Author(s): Adel Zainalpour, Barmak Gholizadeh, Yeganeh Farsi and Tahmineh Mollasharifi*
Primary squamous cell carcinoma (SCC) of colon is a rare histopathologic type of colon cancers. In this article we report a case of a large invasive primary colon SCC mass proven by pathologic examinations at rectosigmoid level with extensive adhesions to vasculature and hydronephrosis due to pressure effects on ureter. We excised the whole mass and dissected all para-aortic and pelvic lymph nodes. Patient was referred to oncology ward for further treatments.
Primary squamous cell carcinoma (SCC) of colon is a very rare histopathologic type of colon cancer which is mainly seen in rectosigmoid junction (1-3). SCC is raised from epithelial cells (2). In this article we report a case of a large invasive primary colon SCC mass at rectosigmoid level with extensive adhesions to vasculature.
A 50-year-old man without any notable past medical history came to surgery ward due to abdominal pain, fullness sensation in abdomen and melena. Further studies revealed a sigmoid mass with extension to retroperitoneal space and iliac vessels with external pressure on the left ureter (Figure 1).
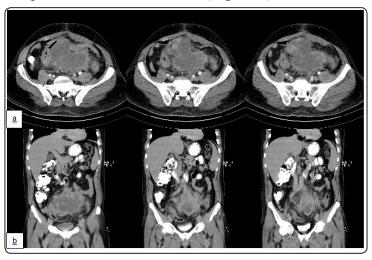
Figure 1: Abdominal CT scan, note tumor and its adhesions to adjacent structures. a) axial view b) coronal view
He underwent surgery in another hospital but due to massive adhesions resection did not placed. The patient underwent laparotomy for the second time in our ward. Tumor presented extensive adhesions to abdominal aorta and iliac vessels but we took a control of infra-renal aorta and dissected it from vessels and ureters carefully. The whole mass and retroperitoneal remnants excised, para-aortic and pelvic lymph node dissection, sigmoidectomy and Low anterior resection was done and primary anastomosis performed (Figure 2).

Figure 2: Surgical procedure. a,b) Extensive adhesion of tumor to abdominal viscera. c) Dissection the mass from adjacent structures. d) Control of Aorta. e,f) Resected tumor
Ureter catheter (DJ) was inserted. Bladder, kidneys and ureters remained intact. Pathologic examination of resected tumor dedicated SCC. Investigation of skin, respiratory system, gastrointestinal system, genito-urinary systems and organ- systems by CT-scans of thorax and abdomen, colonoscopy and endoscopy and laboratory tests did not suggest any primary site for SCC. Based on these information, we considered our patient as a case of primary SCC of colon.
Light microscopic examination of paraffin-embedded sections of sample revealed a malignant tumor composed of polygonal cells with highly pleomorphic nuclei, macronucleoli and abundant eosinophilic cytoplasm arranged in infiltrating nests and sheets. Individual keratotic cells identified, but obvious keratinization was absent. Immunohistochemical demonstration of squamous markers (CK5/6+++, P63+) were in accordance with the diagnosis of poorly differentiated SCC (Figure 3).
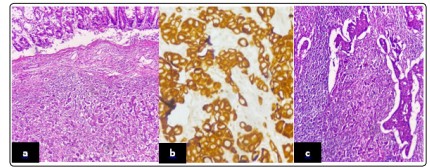
Figure 3: a) Poorly differentiated carcinoma composed of large cells with large nuclei, very irregular, often hyperchromatic without obvious keratinization phenomenon, have abundant cytoplasm (ob. 10×). b) Tumor cells show strong cytoplasmic/memberanous demonstration of CK5/6 (ob. 40×). C) Metastatic lymph node showing metastatic, poorly differentiated SCC (ob. 20×)
The tumor cells were also negative for S100, CK7 and CK20. The margins of the excised tissue were cancer-free with three metastastatic lymph nodes.Primary SCC of colon is so rare that its estimated prevalence is about 3 in every 1000 colorectal cancer patients. The exact etiology, pathophysiology, exclusive clinical features, treatment and prognosis are all unclear due to its rarity (4-5). SCC originates in epithelial cells and mechanisms like malignant transformation of proliferated uncommitted basal cells or preexisting adenomas, differentiation of pluripotent stem cell, metaplasia of epithelial cells due to chronic inflammation, embryonal cloacogenic nests, viral infections, radiation, inflammatory bowel disease all have been proposed but none of them are neither confirmed nor rejected (3, 6-18).
The first reported case of primary SCC of colon was reported in 1919 by Schmidtman (11). Primary SCC of colon is mostly seen in fifth decade of life (19). Most of reported cases have masses in rectosigmoid junction but there are reports of confirmed cases in descending colon too (5,19-21). Primary SCC of colon is indistinguishable from adenocarcinoma of colon clinically as they have the same clinical picture rectorrhagia, abdominal pain, changed bowel habits and weight loss are seen in both of them (1,20). Primary SCC of colons are locally invasive so can present with complications such as bowel obstruction, and urinary obstruction (1,22). Our patient suffered from abdominal pain , melena and left flank pain due to hydronephrosis , but despite his large mass he did not show any bowel obstruction signs or symptoms.
In 1979, Williams et al. established a 4-item criterion to diagnose the primary SCC of colon based on: 1. Exclusion the metastasis from any other primary site 2. Absence of squamous lined fistula in tumoral bowel 3. Ruling out the SCC of anus as the origin with proximal extension 4. Histopathologic confirmation of SCC (1,20). Our patient full-filled these criteria. Prognosis is another question in management of patients with primary SCC of colon. Factors such as ulceration, left side mass, metastasis to lymph node, stage IV and un/poorly differentiation to be associated with poor prognosis (21). Dukes’ staging system for colon cancer estimates the 5-year survival rate about 50% for Duke stage B, 33% for Duke stage C, and 0% for Duke stage D (23).
Tumor markers such as Carcinoembryonic antigen (CEA) and SCC antigen can also be evaluated. CEA seems to be usually within normal range and SCC antigen can be used either to evaluate concomitant tumors or evaluation of recurrence (19,23). Appropriate treatment strategy is also a challenging issue. Surgery, chemotherapy and radiotherapy have been all studied and there is a tendency to do surgery with subsequent chemotherapy (4,11,19,24- 26). Surgical resection is the cornerstone of treatment and its extension depends on tumor size, location, depth of invasion and local/ distant metastasis (1). Local excision, radical excision, Lower anterior resection and abdominoperineal resection all can be considered as potential surgical techniques mainly according to the location of tumor (1,23). There are several studied chemotherapeutic agents but still there is not a consensus about the best option. Efficacy of radiotherapy is also unknown but it might be useful after surgery in patients with positive margins (21). We referred our patient to oncology ward for post operation chemotherapy.
