Author(s): Siva Sathyanarayana Movva
The aim of this research was to evaluate the effectiveness of rainwater treatment using two distinct filtration methods: one utilizing traditional filtration materials (gravel, sand, and anthracite), and the other employing membranes. In both cases, the quality of the treated rainwater met the standards set by NBR 15527:2007 (Brazilian Association of Technical Standards) and the United States Environmental Protection Agency (EPA) for non-potable purposes, including parameters such as pH, temperature, turbidity, ammonia, nitrate, nitrite, alkalinity, and calcium hardness. Furthermore, the findings were compared with Brazilian Ministry of Health Directive 2914/2011, which pertains to water potability, and Resolution 357/2005 of CONAMA (Brazilian National Council for the Environment), addressing surface water bodies, particularly rivers, and permitting direct human skin contact with water. The study concluded that rainwater treated by both filtration methods could be utilized for various non-potable applications such as toilet flushing, garden irrigation, and sidewalk cleaning, as well as for direct-contact activities like bathing and laundry washing.
Water scarcity arises from a combination of factors including the availability and quality of water sources, amplified consumption due to urbanization, human activities, and industrial growth. Addressing the declining quality and scarcity of water requires exploring alternative sources and promoting the prudent use of water to ensure a consistent supply. Rainwater, seen as a potential alternative, is increasingly considered by researchers as a solution to the potable water crisis. Rainwater harvesting has gained popularity, especially in arid or remote areas where conventional water supply systems are impractical or economically unviable. Consequently, rainwater is increasingly recognized as a potential source for both potable and non-potable purposes. Apart from augmenting water supply, rainwater harvesting fosters community engagement in water management, warranting governmental encouragement and support. However, factors like surface characteristics, equipment cleanliness, and roofing material impact the quality of collected rainwater. Contaminants from roof surfaces, including microbial pathogens from bird and animal feces, necessitate proper filtration and treatment. Additionally, the initial runoff from rooftops may contain pollutants in higher concentrations, underscoring the importance of installing first flush diverters. Filtration processes, employing physical, chemical, and sometimes biological methods, aid in removing suspended particles and microorganisms from rainwater. Sand filters, for instance, effectively capture solid matter, algae, and organic particles, contributing to improved water quality. Moreover, membranes offer selective filtration based on molecular weight, with various types such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, each serving specific purposes. Research suggests combining sand filtration with activated carbon technology can effectively remove pathogens, turbidity, and odor from rainwater. Several studies have explored filtration systems utilizing sand, activated carbon, and membranes for rainwater treatment, demonstrating their efficacy in producing potable water. The objective of this study is to compare the efficiency of rainwater treatment using two filter types: one comprising gravel, sand, and activated carbon, and the other utilizing needle-punched nonwoven polyester geotextile membrane.
Two types of filtration systems were developed for rainwater treatment and subjected to comparative evaluation. The first system utilized a downward flow sand filter, while the second employed a membrane filter. Both filters were deployed side by side in São José, a city situated in the state of Santa Catarina, southern Brazil. This arrangement ensured that they were exposed to identical rainwater conditions in terms of quality and intensity. The installation site, situated approximately 100 meters from highway BR 277, experiences significant traffic flow, and is about 200 meters away from the sea. The location of the installation is depicted in Figure 1 file.
Figure 1: Location of Site for Installation of Filters for Rainwater Treatment.
The catchment area measured 50.70 square meters and comprised fibrocement roof tiles, gutters, pipes, a first flush device, and a reservoir. The system operates via gravity in the following sequence: water flows over the roof, captured by the gutter. Prior to treatment, the water passes through a screen to filter out any coarse material such as twigs and leaves. It then travels through a piping system to the first flush device, which discards the initial two millimeters of rainwater. Once the first flush device reaches capacity, water is directed through the filter via a T-connection. Following filtration, the water proceeds to a reservoir consisting of a 310-liter water tank. The first flush device is designed to retain the first two millimeters of rainfall. However, in this study, a 2-mm hole was incorporated at the end of the first flush device’s pipe for automatic drainage. It was observed that this hole frequently became clogged due to dirt present in the atmosphere and on the roof, brought in by the rainwater. Consequently, manual cleaning was necessary after each rainfall event. Further details regarding the sand filter are illustrated in Figure 2.
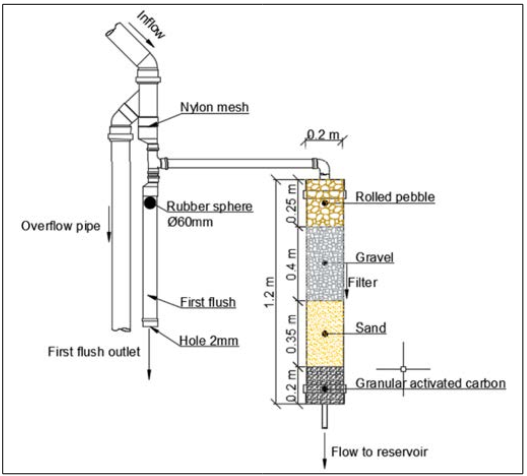
Figure 2: Schematic Showing the Sand and Filter
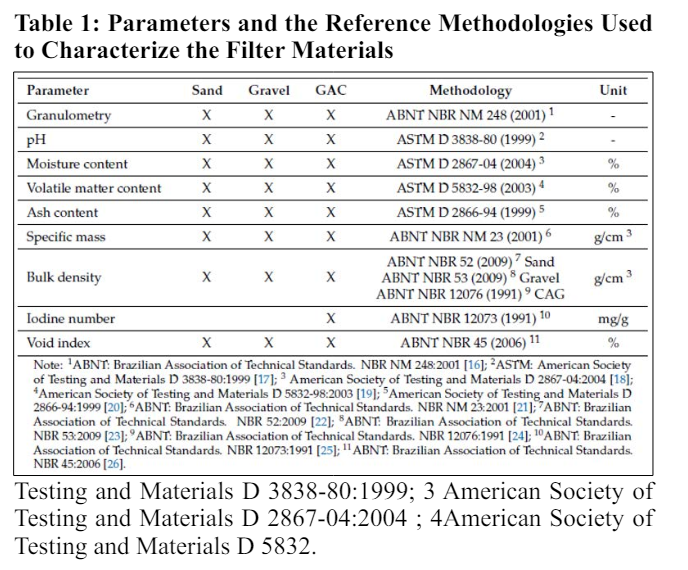
The downward flow filter was made of PVC and had a support layer 0.25 meters thick with rolled pebbles ranging from 2.5 to 3.8 centimeters in diameter. It also included a filtration layer consisting of 0.40 meters of gravel, 0.35 meters of sand, and 0.20 meters of GAC (see Figure 3). Before construction, sand, gravel, and rolled pebbles were washed with water and dried in an oven at 100°C for 24 hours to reduce initial turbidity and speed up maturation. Carbon activation involved heating in an oven at 300°C for 24 hours. Various tests were conducted on the filtration materials including granulometry, void index, specific mass, pH, volatile matter content, ash content, moisture content, bulk density of sand, gravel, and activated carbon, and determination of the iodine number of the activated carbon. Tests were performed at the Soil Mechanics Laboratory at UTFPR, Campus Ecoville. See Table 1 for details on the methodologies used.

Figure 3: Materials used in the Filter: (a) Rolled Pebbles; (b) Gravel; (c) Sand; (d) Granular Activated Carbon (GAC).
This study evaluated the efficiency of a membrane filter system using needle-punched nonwoven polyester geotextile (Bidim RT31) in rainwater harvesting. The membrane had hydraulic properties: permeability (kn) = 0.37 cm/s, permittivity = 0.8 s^-1, and apparent opening size (O95) = 0.125mm. The model assessed was based on Vieira et al.’s proposal. Figure 4 illustrates the operational stages and key materials for the filter.
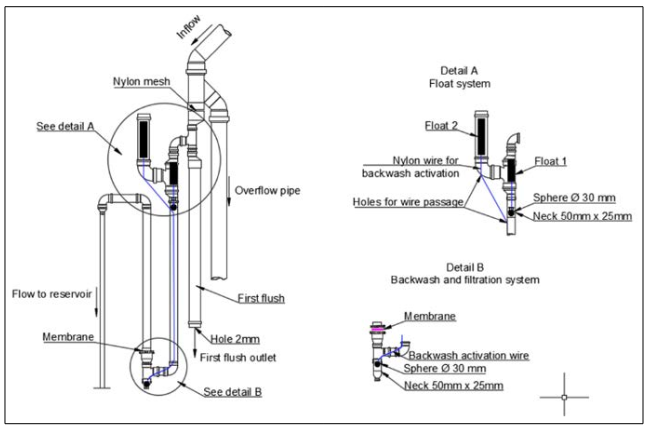
Figure 4: Schematic Showing the Assembly of the Membrane Filter System
The membrane filter was designed to carry out the backwashing process automatically and mechanically, without requiring manual operation. The system works as follows. The water that flows through the gutter fills up the first flush device and then enters the treatment system. The first pipe is filled with water and has a downward flow direction, while the second pipe has an upward flow direction and the water passes through the filtration membrane. After filtration, the water flows to the reservoir, which is totally sealed and does not allow water to leak. In this case, a water storage tank of 310 L with a threaded lid was used.
When the whole system is filled with water, float 1 rises inside the pipe and a rubber sphere with a diameter equal to 30 mm (sphere 1) interrupts the inward flow of the filtration system. At this moment, the rainwater is directed to another set of pipes containing float 2, which is then set in motion and rises within the pipe. Float 2 is connected, through a steel cable, to a second rubber sphere with a diameter equal to 30 mm (sphere 2), which is placed at the connection below the filtration membrane. Thus, when float 2 rises within the pipe, the steel cable pulls sphere 2. The water flow in the pipe is thereby released and carries out the backwashing of the filtration membrane. The two floats were built from pieces of PVC pipes with a diameter equal to 50 mm, closed at the ends with caps with the same diameter.
Physicochemical parameters were carefully selected for comparative analysis to understand contaminant levels in rainwater treatments using sand and membrane filters. These parameters included pH, temperature, turbidity, alkalinity, calcium hardness, as well as concentrations of ammonia, nitrite, nitrate, and phosphate. The assessment aimed to evaluate treatment effectiveness and compile a rainwater quality database, comparing parameter values against established water quality standards. Methods for analyzing these parameters followed Standard Methods for Examination of Water and Wastewater, with a multiparameter bench photometer (Hanna HI 83099) utilized for analysis.
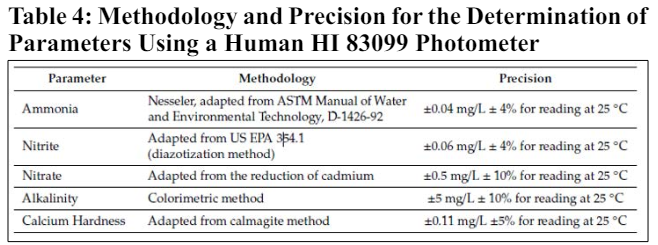
The sample collection was conducted approximately twice per month, dependent on rainfall frequency. A total of 15 collections took place between December 2015 and August 2016. Water samples were collected from rainwater storage reservoirs installed after each filter. Samples were taken about 10 cm below the water surface to avoid disturbing sediment. Untreated rainwater samples were also collected from a separate container. All samples were promptly stored in sterilized containers and kept on ice until analysis. The experimental data is presented in box- plot graphs, showing main trends and data variability. They include the median (50th percentile), lower (25th percentile), and upper (75th percentile) quarters, along with measures like minimum and maximum values. The results were compared with Resolution 357/2005 of the Brazilian National Council for the Environment, which provides water body classifications and environmental guidelines, as well as discharge standards. Additionally, comparisons were made with the values defined by the United States Environmental Protection Agency (EPA), which establishes guidelines for water and rainwater use. The results were compared with Brazilian Ministry of Health Directive 2914/2011, which sets guidelines for water quality and potability, and NBR 15527:2007 by the Brazilian Association of Technical Standards, which outlines requirements for non-potable use of rainwater collected from urban roofs. Association of Technical Standards), which establishes the requirements for non-potable use of rainwater collected from roofs in urban areas. According to NBR 15527:2007, rainwater must go through a disinfection process, which may be chlorination, ultraviolet rays, ozone, or other processes, defined at the discretion of the designer The parameters measured in this research are sufficient to guarantee non-potable use, considering that rainwater must go through a disinfection process after filtration. A comparison of the rainwater quality with the values given in CONAMA Resolution 357/2005 was also carried out in order to establish which treatment is suitable for obtaining water for potable use.
The daily rainfall from 01/12/2015 to 07/08/2016 was analyzed. 130 days had no rain during this period. The highest precipitation was 93.4 mm on 03/03/2016. Figure 5 illustrates the daily rainfall pattern for São José, including average daily rainfall and rainless days. Data sourced from the National Institute of Meteorology (INMET).
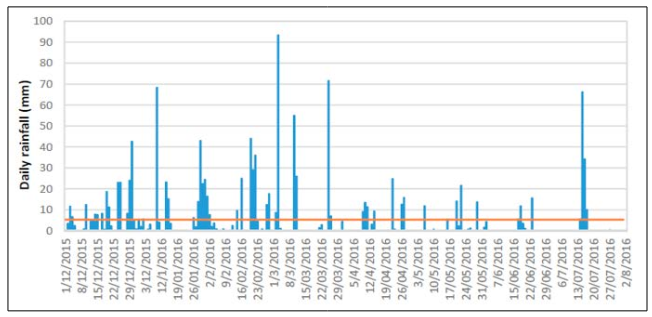
Figure 5: Daily Rainfall in the City Sao Jose from 01/12/2015 to 07/08/2016
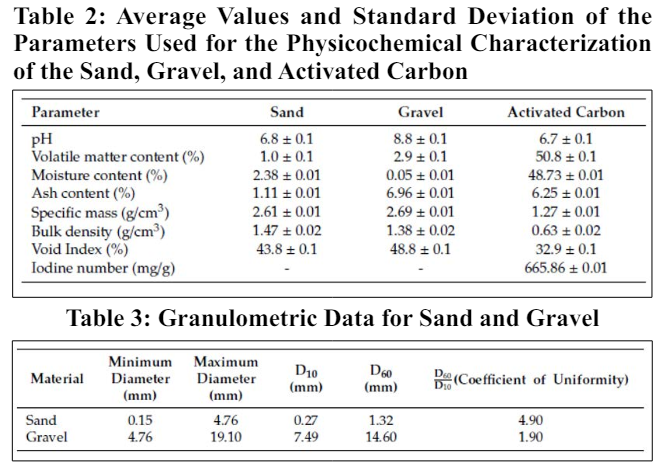
The incorporation of impurities present on the collection surface into the rainwater occurs due to the direct contact of rainwater with the roof. Since the installation site of the two rainwater harvesting treatment filters is situated approximately 100 meters away from a heavily trafficked highway, an examination of pH data confirmed that the rainwater fell within the normal acidity range and did not qualify as acid rain (i.e., pH < 5, which was not observed). Figure 6 depicts box-plot graphs illustrating the median, minimum, maximum, first and third quartiles, as well as the interquartile range of the assessed parameters. The sand and gravel filtration materials used in the sand filter demonstrated a high level of uniformity, with a uniformity coefficient of 4.9 for the sand and 1.9 for the gravel. This level of uniformity closely aligns with findings by Sezerino [34], who reported coefficients of uniformity of 5.70 for sand and 1.89 for gravel during the characterization of filtration materials.
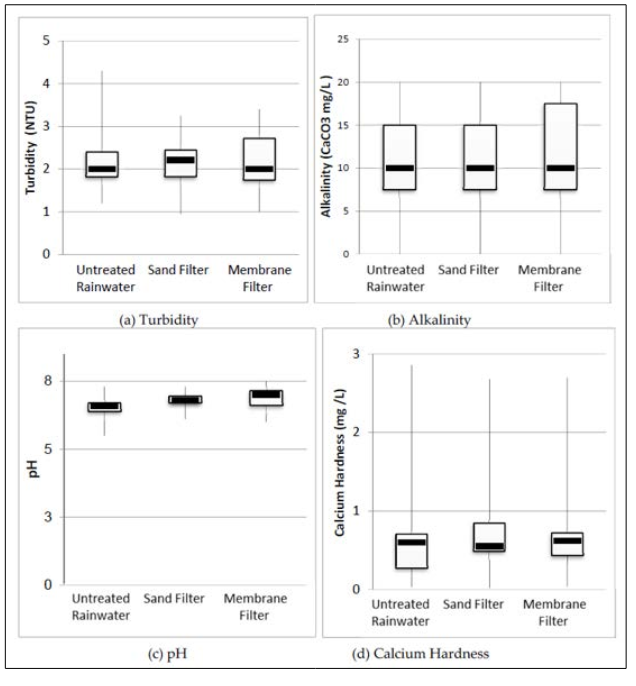
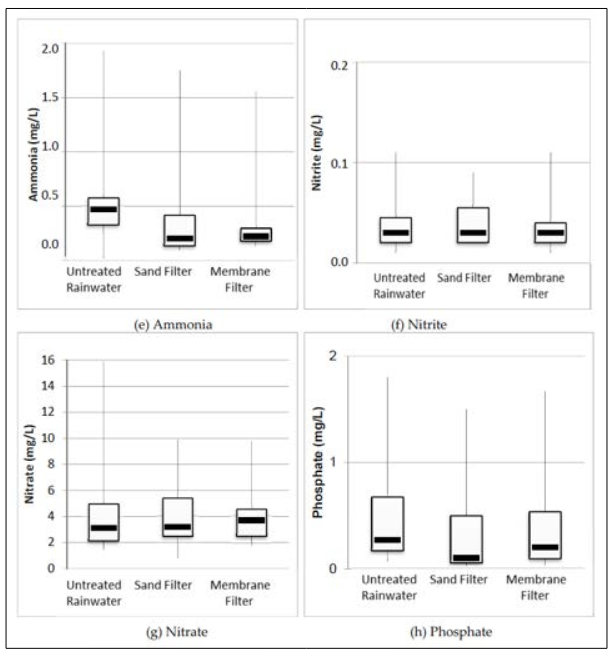
Figure 6: Box-plot with Median, Minimum, Maximum, 1st and 3rd Quarters, and Interquartile Range of the Parameters Evaluated.
(a) Turbidity
(b) Alkalinity
(c) PH
(d) Calcium Hardness
(e) Ammonia
(f) Nitrite
(g) Nitrate
(h) Phosphate
According to Sezerino, a coefficient of uniformity lower than or equal to 5 is recommended for sand. Coelho and Di Bernardo obtained coefficients of uniformity equal to 2.0 and lower than 1.7 for sand and granular activated carbon, respectively. Brinck obtained coefficients of uniformity equal to 1.76 and 1.97 for different sands and 1.30 and 1.96 for different anthracites. The lower the value for the coefficient of uniformity, the more uniform the granular material, the deeper the penetration of impurities, and the longer the filtration run time will be.
Table 5 provides a statistical summary of the results for the physicochemical analysis of the rainwater collected from the sampling points and allows a comparison with the values established in guidelines for water quality. In Brazil, that is, CONAMA Resolution 357/2005, NBR 15527:2007, and MS Directive 29414/2011. The results were also compared with the values given in the US EPA.
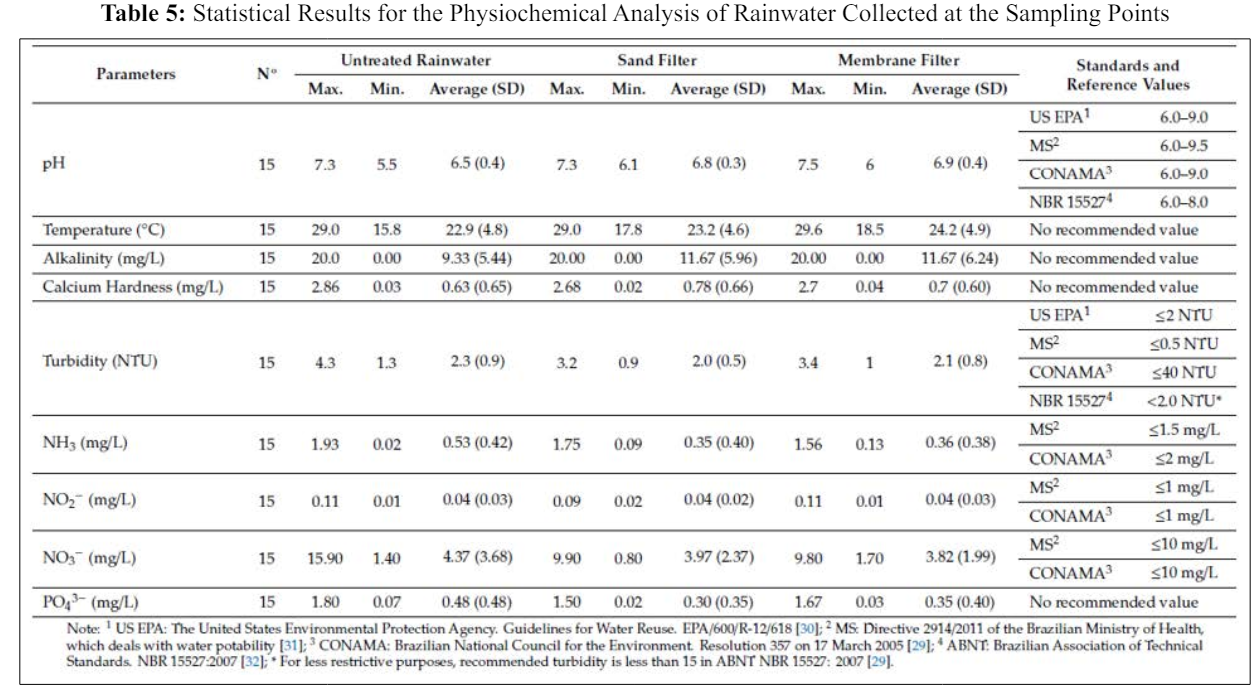
Table 5 illustrates that the pH levels of rainwater samples post- treatment met the prescribed standards in terms of both average and standard deviation. As for ammonia and nitrite concentrations, they consistently adhered to the limits outlined in CONAMA Resolution 357/2005 for class 2 water bodies, despite this resolution being intended for a different water body type; it served as a benchmark for comparison purposes in this study. However, the turbidity readings exceeded those specified in NBR 15527:2007, particularly for untreated rainwater, possibly attributable to the building’s proximity to highway BR 277. The turbidity findings indicate that both filters effectively trapped particulate matter, resulting in reduced turbidity levels. Notably, the highest turbidity readings for untreated rainwater and filtered water were recorded on 28/06/2016 and 13/07/2016, following prolonged periods of no rainfall, which facilitated the accumulation of atmospheric particulate matter, consequently elevating rainwater turbidity.
Based on MS Directive 2914/2011, the highest ammonia levels exceeded limits, while nitrite levels were acceptable. For nitrate, only untreated rainwater surpassed limits set by MS Directive 2914/2011 and CONAMA Resolution 357/2005. Filters efficiently removed ammonia but not nitrite. No specific limits were provided by US EPA or NBR 15527:2007 for ammonia, nitrite, and nitrate. pH levels at all collection points met guidelines. Turbidity was within limits only after passing through filters, while untreated rainwater turbidity met only CONAMA Resolution 357/2005. Average ammonia, nitrite, and nitrate values complied with MS Directive 2914/2011 and CONAMA Resolution 357/2005, not addressed by US EPA or NBR 15527:2007. Honório et al. conducted a study in the western Amazonia, Brazil, assessing untreated rainwater quality. Nitrate concentrations ranged from They found pH 5.3 and nitrate 2.2 mg/L for roof rainwater, and pH 7.8 and nitrate 7.6 mg/L for reservoir rainwater. The study didn’t assess physicochemical parameters of the first flush device, but observed clogging of the self-cleaning 2-mm hole due to accumulated dirt, indicating impurities. Silva found first flush devices more effective than filters in removing impurities. Sand filters removed 13.0% turbidity, 34.0% ammoniacal nitrogen, and 10.0% nitrate; membrane filters removed 11.0%, 32.1%, and 13.6%, respectively.
The treatment didn’t significantly affect pH and nitrite levels in both filters due to initial pH neutrality and low nitrite concentrations in untreated rainwater. The sand and gravel filter remained efficient without backwashing, while the membrane filter required membrane replacement after four months to maintain efficiency. Overall, both filters showed similar efficiency, but the sand filter proved more suitable due to its maintenance-free performance throughout the study period, contrasting with the membrane filter’s need for adjustments and replacement to sustain efficiency. Therefore, for the evaluated period, the sand filter emerged as the preferred option for rainwater treatment.
This study assessed two rainwater filters’ efficiency. In a region with already good rainwater quality, both filters performed similarly, with no significant difference. However, in areas with lower quality rainwater, filters can be more effective. First flush devices are crucial for rainwater treatment, as they discard initial impurities carried by rainwater flowing over roofs, helping maintain filter systems’ cleanliness and safety. The study observed impurity accumulation in these devices for both filters. Overall, the tested rainwater treatment systems were effective, highlighting rainwater’s potential as a water source alongside utilities, reuse, and underground sources. In regions with good rainwater quality, first flush devices are essential, and the need for filtration depends on rainwater quality. Even with high-quality treated rainwater, non-potable use requires disinfection for user safety.
1. Naddeo V, Scannapieco D, Belgiorno V (2013) Enhanced drinking water supply through harvested rainwater treatment. J Hydrol 498: 287-293.
2. Lee YJ, Yang JS, Han M, Choi J (2010) Comparison of the microbiological and chemical characterization of harvested rainwater and reservoir water as alternative water resources. Sci Total Environ 408: 896-905.
3. UNESCO (2010) United Nations World Water Development Report: Leaving on One behind Facts and Figures 2019 https://unesdoc.unesco.org/ark:/48223/pf0000367276.
4. Wilbers GJ, Sebesvari Z, Rechenburg A, Renaud FG (2013) Effects of local spatial conditions on the quality of harvested rainwater in the Mekong Delta-Vietnam. Environ Pollut 182: 225-232.
5. Weisbeck E, Sandri EK, Soares ALM, Medeiros SHW (2011) Disinfection of rainwater by ultraviolet radiation. sanitary and Environmental Engineering 16: 337-342.
6. Barret BC, Kinney AK, Kirisits JM (2011) The effect of roofing material on the quality of harvested rainwater. Water Res 45: 2049-2059.
7. Gikas DG, Tsihrintizs AV (2012) Assessment of water quality of first-flush roof runoff and harvested rainwater. J Hydrol 466-467.
8. Coelho ERC, di Bernardo L (2012) Removal of atrazine and metabolites by slow filtration with a bed of sand and granular activated carbon. Sanitary and Environmental Engineering 17: 269-276.
9. Brinck NCP (2009) Assessment of the type of filtering material on the hydraulic behavior of deep-layer rapid filters in the treatment of supply water. Ph.D. Thesis, Escola Politécnica da Universidadede São Paulo, São Paulo, Brazil, 2009. (In Portuguese).
10. Testezlaf R (2008) Sand filters applied to localized irrigation: Theory and Practice. Agricultural Engineering 28: 604-613.
11. Aslan A (2005) Combined removal of pesticides and rates in drinking waters using biodenitrification and sand filter system. Process Biochem 40: 417-424.
12. Zhang G, Yang Y, Liu X, Zhao W (2011) Research and application of harvested rainwater in the villages and towns of China Loess Plateau region. Energy Procedia 5: 307-313.
13. Shaheed R, Mohtar WHMW, El Shafie A (2017) Ensuring water security by utilizing roof-harvested rainwater and lake water treated with a low-cost integrated adsorption-filtration system. Water Sci Eng 10: 115-124.
14. Yan X, Wards S, Butler D, Daly B (2018) Performance assessment and life cycle analysis of potable water production from harvested rainwater by a decentralized system. J Clean Product 172: 2167-2173.
15. Tang X, Pronk W, Ding A, Cheng X, Wang J, et al. (2018) Coupling GAC to ultra-low-pressure filtration to modify the biofounling layer do bio-community: Flux enhancement and water quality improvement. Chem Eng J 333: 289-299.
16. ABNT (Brazilian Association of Technical Standards). Aggregates—Determination of granulometric composition— NBR NM 248:2001; ABNT: Rio de Janeiro, Brazil, 2001. (In Portuguese)
17. ASTM- American Society of Testing and Materials. D 3838-1980: Standard Test Method for pH of Activated Carbon; American Society of Testing and Materials: West Conshohocken, PA, USA, 1999.
18. ASTM- American Society of Testing and Materials. D 2867-2004: Standard Test Method for Moisture in Activated Carbon; American Society of Testing and Materials: West Conshohocken, PA, USA, 2004.
19. ASTM- American Society of Testing and Materials. D 5832- 1998: Standard Test Method for Volatile Matter Content of Activated Carbon; American Society of Testing and Materials: West Conshohocken, PA, USA, 2003.
20. ASTM- American Society of Testing and Materials. D 2866-1994: Standard Test Method for Total Ash Content of Activated Carbon; American Society of Testing and Materials: West Conshohocken, PA, USA, 1998.
21. ABNT (Brazilian Association of Technical Standards). Portland Cement and Other Powdered Materials— Determination of Specific Mass—NBR NM 23:2001; ABNT: Rio de Janeiro, Brazil, 2001. (In Portuguese)
22. ABNT (Brazilian Association of Technical Standards) (2009) Fine Aggregate—Determination of Specific and Apparent Mass—NBR 53:2009; ABNT: Rio de Janeiro, Brazil (In Portuguese).
View PDF