Author(s): Nádia Isaac da Silva and Ivo Lebrun*
The article narrates a brief history about the first possible descriptions of autism spectrum disorder (ASD) until the publication of Leo Kanner and Hans Asperger, then reports the evolution of its definition and concept, contextualizing its first inclusion in the ICD (International Statistical Classification of Diseases and Health related problems) and DMS (Diagnostic and statistical Manual of mental disorders) up to its latest versions. It discusses the clinical picture characterized by heterogeneous manifestation and associated comorbidities. This article also presents the weight of the contribution of genetic and environmental factors in the etiology of the disorder, t he diagnosis based on clinical observation and absence of biomarkers, discussions th and efficacy and limitation of pharmacological treatment and pedagogical approaches, similarly exposes a possible research strand for ASD, developed from the production of biobanks as a source of investigation of symptoms, genetic alterations, physiological dysfunctions with the objective of identifying subgroups of individuals with ASD and thus enable the targeting of individualized and more effective interventions.
In the psychiatric literature, since the beginning of the eighteenth century, descriptions of isolated cases of which are now recognized as autism are found. Although some descriptions are very rich and denote psychiatric disorders that shared autistic characteristics, no scholar has seen connections between individual cases until the last half of the 19th century [1,2].
Henry Maudsley in 1867 suggested that children with “very strange” behavior could be classified as having some type of childhood psychosis. Initially, this idea was a shock to society at the time, however, several researchers began to describe and group children and adolescents with some type of “bizarre” behavior [1,2].
In 1906, Plouller introduced the adjective autism in psychiatric literature, when studying patients who were diagnosed with early dementia, but it was Bleuler, in 1911, the first to spread the term autism, as a basic disorder of schizophrenia characterized by a limitation of personal relationships and with the external world, seeming to exclude everything that seemed to be the person’s “I” [3].
The term “autism”, applied to schizophrenia, has spread rapidly in european medical literature. In Germany, in 1920, Künkel referred to a group of schizophrenic children as “autistic”. These children in his description were restless, closed and lonely and with good intellectual development. Russian psychiatrist Grunja Jefimov Ssucharewa (1926) adopted the concept of “autistic attitude” to name the behavior observed in six children with schizoid personality disorder. This attitude was characterized by social isolation, accompanied by strange thoughts (perseverance, rumination and rationalization), preference for fantasy stories and fairy tales, emotional dysregulation, presence of echolalia, impulsivity and stereotyped behavior. Later, Potter (1933) and Despert (1938) described children with affective relationship problems and other traits of autistic behavior as more interested in the form of words than in their communicative function, restricted interest and repetitive movements [4,5].
In the year 1943, two descriptions of children with severe and unusual social deficits were published. The authors used the term “autistic” to designate a clinical condition that differed from schizophrenia and childhood psychosis. These publications are considered the first relevant descriptions of autism.
Psychiatrist Leo Kanner, in Baltimore, USA, systematized the careful observation of a group of eleven children aged between two and eight years, in his article “Autistic Disturbance of Affective Contact”. In his work, he claimed to have identified a rare condition with symptoms present since early life. He described behavioral changes that were repeated and remained unchanged over time as: complete attachment to daily routines, extreme isolation and preference for inanimate objects over people. He also reported delay in language acquisition, non-communicative use of language after its development, tendency to repeat the speech of the other (echolalia), reverse use of pronouns, repetitive and stereotyped games, lack of imagination, good mechanical memory and appearance normal physics. All of the characteristics described were often associated with intellectual disability, or fragmented intellectual development. In the year 1944, Kanner called this set of signs and symptoms “early childhood autism”. The work had great relevance for the construction of the concept of autism because it was published in the United States, where it reached a notable diffusion in the field of child psychiatry [5-8].
Hans Asperger, unaware of Kanner’s work, defended his thesis entitled “Die ‘Autistischen Psychopathen’im Kindesalter”, which was published in 1944. In it he reported cases of four boys between the ages of seven and eleven. He claimed that “autistic psychopathy” was a common condition, but rare in girls. The patients identified by Asperger showed a pattern of conduct similar to that of Kanner’s patients, except for the preservation of verbal and cognitive ability. The clinical picture was characterized by a lack of empathy, naivety, little ability to make friends, pedantic and repetitive language, poor non-verbal communication, exaggerated interest in a certain topic, motor inability and lack of coordination. In fact, since all of his works were written in German during the Nazi era, they were largely ignored by psychiatry and neurology in most countries. Recognition began around 1981, after the publication of translations (German to English) of his works by Lorna Wing. In return for his work, the condition he described was named Asperger’s Syndrome [4, 9-11].
During the fifties and sixties, there was much confusion about the nature of the disorder and its etiology. During this period, one of the main lines of research focused on the belief that non-emotionally responsive parents were responsible for the disorder in their children (the “refrigerator mother” hypothesis). Concomitantly, there was a strong movement that considered autism as the first manifestation of childhood psychosis or schizophrenia. For this reason, for a long time, the concepts of autism, psychosis and schizophrenia were confused and used interchangeably [12,13]. In the 1960s there was an increase in the number of scientific evidences that autism was a neurobiological disorder. At the end of the seventies, the first studies with twins had a strong genetic basis for the condition.
A major step towards the classification of autism occurred after the publication of the studies by Kolvin (1972) and Rutter (1972). In them, the authors proved that autism was different from schizophrenia (even in childhood schizophrenia) in terms of appearance, clinical characteristics and family history. At the same time, a consensus began to develop on the importance of studying autism regardless of schizophrenia. A series of studies attempted to update Kanner’s definition with more formal diagnostic guidelines [3,11].
Only in the early 1980s did autism receive its official recognition, as a pathology distinct from schizophrenia and with its own evolutionary context. Autism was removed from the category of psychosis in DSM-III and ICD-10, becoming part of invasive developmental disorders (TID) together with invasive developmental disorder with no other specification (TID-SOE) [14].
From 1981, with the dissemination of Asperger’s work by Lorna Wing, the focus of the investigations was on high functioning individuals. This fact allowed the construction of the concept of “spectrum” of autism, which proved to be useful both in the clinical field and in genetic research [7]. Only in 1987, in the revised edition of DSM-III-R, was the concept of autism spectrum disorder (ASD) registered. In 1994, DSM-IV brought autism as a member of the TID, along with TID-SOE, childhood disintegrative disorder, Rett syndrome and Asperger’s syndrome. Both ICD-10 and DSM-IV established commitment in three main areas as a criterion for autism: qualitative changes in reciprocal social interactions; communication modalities; restricted, stereotyped and repetitive activities and interests. Based on the changes mentioned, the DSM-IV and ICD-10 classification systems became equivalente [12,14-16].
The fifth edition of the DSM-V was published in May 2013 and brought about a major change in the concept of autism. In this last version, with the exception of Rett syndrome, the disorders belonging to the TID, which had a distinct diagnosis, were condensed into a single diagnosis, the “autism spectrum disorder” (ASD). The edition also brought a new symptom structure, and the triad of symptoms that modeled communication deficits separately from DSM-IV social impairments, was replaced by a three-domain model composed of a domain related to social communication deficit, the according to restricted and repetitive behaviors and interests, and the third integrated by sensory sensitivity. The distinction between cases is made according to the level of severity that each domain was affected [11,17].
In the year 2022 the version of the DMS-V-TR was published and in general no changes were made there was only one change from the original manual: now, to fit into an autism picture, people need to fit all the sub-characteristics of the social communication difficulty domain [18]. In 2022, the World Health Organization published the latest version of the International Statistical Classification of Diseases and Related Health Problems (ICD-11), this new version also followed the change made in DMS V. The document merged all disorders that were within the autism spectrum into a single diagnosis of ASD. In ICD-11, ASD is identified by code 6A02, replacing F84.0, and the subdivisions are now related to the presence or absence of intellectual disability and/or functional language impairment [12,19]. Figure 1 illustrates the evolution of the diagnosis of ASD [20].
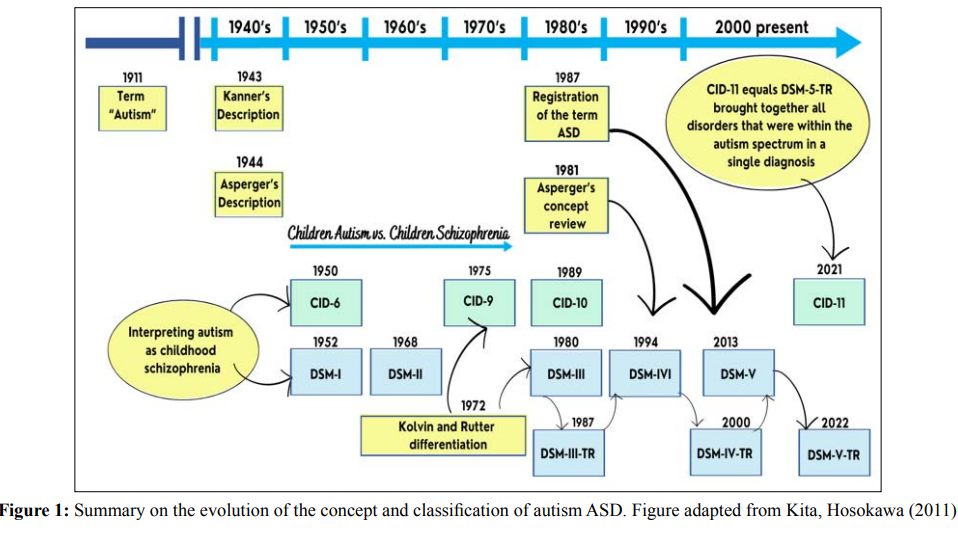
ASD is a developmental disorder characterized by a peculiar behavioral picture and that always involves the areas of social interaction, communication and behavior. The disorder, being a spectrum of conditions, manifests in a heterogeneous way, which implies clinical manifestations with great variability in the intensity of the damage present in each area of impairment [21].
Symptoms usually appear before the age of three. In early childhood, there is a tendency to attach to unusual, typically rigid objects. The child tends to insist on the performance of particular routines and non-functional rituals. In some cases, there are interests such as: dates, itineraries and motor stereotypes. In addition to these specific diagnostic aspects, children with ASD often demonstrate a number of other non-specific problems, such as fears, phobias, tantrums and aggression. Losses in communication and language are frequent and, in general, severe. Other common symptoms also refer to hyper or hyporeaction to sensory stimuli, such as light, pain or sound. Many children are unable to identify situations of real danger such as moving vehicles or great heights [3, 22].
Severe conditions coexist with the TEA picture. Intellectual disability is present in varying levels of severity in approximately 30% to 50% of cases and more than half of this population suffers from a psychiatric disorder such as bipolar disorder, depression, anxiety, attention deficit disorder and hyperactivity [23]. Some medical conditions are also present, including: sleep and eating disorders, epilepsy, metabolic changes related to proteins and lipids, mitochondrial dysfunction, abnormalities of the digestive tract (GIT) and immune system, hearing and visual deficiencies. There are also cases of ASD identified coexisting with other pathologies such as Down syndrome, cerebral palsy, fragile X syndrome, Tourette syndrome [22]. Figure 2 illustrates the clinical picture of ASD, which is composed of the central symptoms of the disorder and several medical conditions [24].
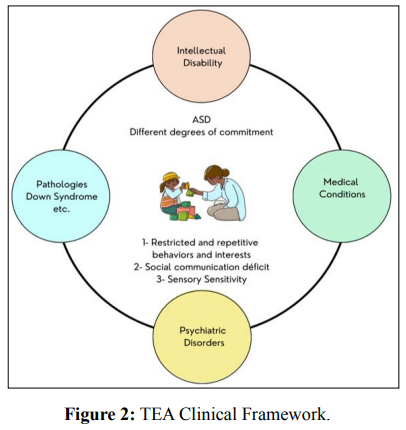
Studies on the prevalence of ASD present heterogeneous and inconsistent results. To date, no epidemiological work has been supported to explain differences in prevalence between geographic regions or variability based on ethnicity or socioeconomic factors [22].
A systematic review by Salari and colleagues (2022) identified a prevalence of ASD worldwide of 0.6%. Subgroup analyses indicated that the prevalence of ASD in Asia, America, Europe, Africa, and Australia was 0.4%, 1%, 0.5%, 1%, respectively [25]. In Brazil, a pilot study carried out in the city of Atibaia, in the interior of the state of São Paulo, points to an estimate of 27.2/ 10,000 [26]. Currently, there is a consensus on the alarming increase in prevalence over the past two decades. In 2000, the CDC(Centers for Disease Control and Prevention) started monitoring the prevalence of ASD in eight-year-olds in six to eleven USA states. Initial reports estimated the prevalence rate to be 6.7 / 1000, but the latest published in 2020 points to an index of 18.6/ 1000 [27,28].
According to the literature, the increase in prevalence is related to a combination of factors such as the adoption of broader definitions of ASD, greater awareness among specialists and the non-medical community about the different manifestations, better detection of cases without mental retardation and availability of specialized care services [12,20]. Some studies associate this fact to the increase in maternal prenatal or postnatal exposure to environmental pollutants, tobacco and alcohol [29,30].
The male sex is more affected, with an average ratio of 4 to 5 men to 1 woman. Bedford et al (2016) in their research observed that some predictors of autism in the first year of life (speed of attentional disengagement and variation in gaze tracking etc.), more strongly predicted ASD in males than in female siblings of probands with ASD [31]. Research suggests that this peculiarity between sex is mediated by biological factors that are still unknown [32].
The heterogeneity of the clinical entities that make up the TEA is the result of a complex interaction between genes of multiple and variable susceptibility, epigenetic effects and environmental factors [33,34]. Family and twin studies show the genetic basis of the etiology of ASD: the agreement rate between monozygotic twins is high (60% - 90%) compared with the agreement rate between dizygotic twins (5% -40%) [35,36]. The risk of recurrence between siblings is 12.9% to 18.7% and heritability is estimated to be between 40% -80% [37,38]. Some studies with twin brothers show more conservative results for heritability with moderate heritability 21%-38% [39,40].
Despite the recognition of the weight of the genetic factor on the etiology of ASD, only 10 to 20% of individuals have the etiologic mechanism identified [41]. The genetic architecture of the disorder is highly heterogeneous, resulting from the interaction of complex genetic factors, which are composed of different forms of genetic variation, including chromosomal abnormalities, variation in the number of copies (CNV - copy number variation ); monogenic disorders, single nucleotide polymorphisms (SNP - single nucleotide polymorphism), de novo mutations and epigenetic processes [24]. Case-control studies in population and animal models have identified more than 800 genes associated with autism. The genes most affected in ASD code for proteins involved in chromatin remodeling and transcriptional regulation, cell proliferation, and especially synaptic architecture and functionality [42].
Twin studies provide strong evidence on the weight of the contribution of environmental and genetic factors to the development of the disorder. Hallmayer et al. (2011) observed 192 pairs of twins and concluded that 55% of the risk of the disorder is of environmental responsibility and 37% of genetic factors [39]. A similar result was also found in a large independent study based on the Swedish National Population Register. In a sample of 2.049.973 siblings, which included dizygotic and monozygotic twins, they estimated the etiological responsibility to be 50% for heritability and 50% for unshared environmental influence [37].
The nature of the environmental trigger is still controversial, studies on risk factors associated with ASD in the prenatal, perinatal and neonatal periods identified gestational diabetes , maternal hypertension and proteinuria, pre-eclampsia, gestational bleeding, maternal and advanced paternal age during conception, maternal migration, abnormal fetal presentation, umbilical cord complications, low Apgar score / 5-min, low birth weight (<1500g), meconium aspiration, neonatal anemia, ABO or Rh incompatibility and hyperbilirubinemia, maternal folic acid deficiency during perinatal and gestational periods [43,44]. Research has also reported a consistent association of ASD with exposure in utero to known teratogenic drugs, thalidomide and valproate, or the abortion misoprostol [45-48].
Exposure to intrauterine infections was associated with a significant increase in risk for ASD in studies controlled by multiple covariables or that used siblings as controls [39]. Currently, researchers describe two possible mechanisms: the direct action of the infectious agent or the action of the maternal immune system on the fetal central nervous system (CNS) [49].
Currently emerging environmental factors related to the disorder are being studied, including early maternal prenatal or postnatal exposure to environmental pollutants (heavy metals, fertilizers, organophosphate pesticides, traffic pollutants), maternal exposure to tobacco and alcohol, maternal obesity and paternal, high maternal weight gain during pregnancy, maternal intake of a diet rich in saturated fatty acids and deficient in omega - 3 and vitamin D during pregnancy [34,41,50].
Environmental risk factors act by disrupting the genome as well as the epigenome of neurons or biological pathways that affect the development of the central nervous system (CNS). Imaging studies and autopsies indicate dysregulation of neurogenesis, migration and neuronal maturation. The development process is vulnerable in the prenatal period (first trimester) and in the early postnatal period. Within these periods the highest susceptibility windows vary according to the agen environmental [29,40,41]. Figure 3 shows a summary that includes some environmental factors with their respective susceptibility windows [30].
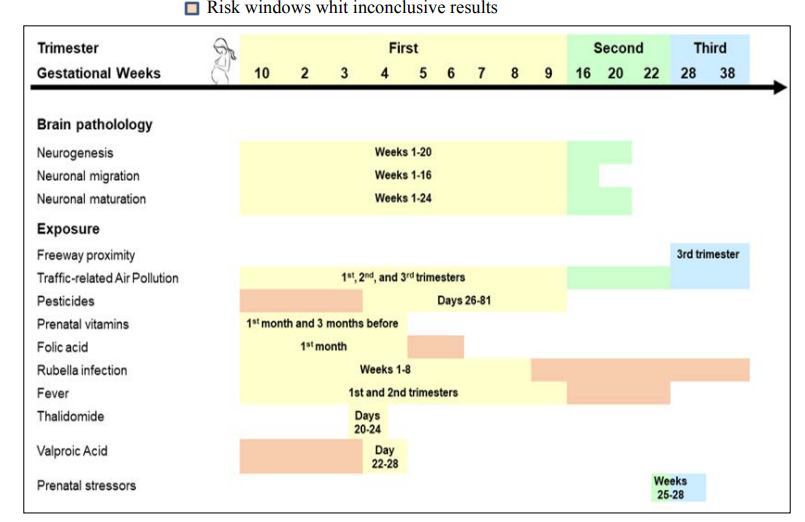
Figure 3: Environmental risk factors for TEA and their respective susceptibility windows. Figure adapted from Lyall, Schmidt Hertz-Picciotto (2014).
The diagnosis is fundamentally clinical and based on the presence of the aforementioned symptoms. The most used classification manuals are the DSM-5 -TR Diagnostic Classification Manual of the American Academy of Psychiatry, launched in 2022; and ICD- 11 - World Health Organization International Code of Diseases, published in 2022 [18,19].
To date, there are no biological markers or endophenotypes that define the diagnosis of ASD. The assessment requires a multidisciplinary team and the use of a series of instruments with the objective of a greater standardization of the way of diagnosing among professionals with different backgrounds and levels of knowledge on the subject. These instruments should be used to assess both the social behavior of children (joint attention, eye contact, facial expression of affection) and their ability to imitate [51,52]. After diagnosis, a careful clinical assessment with language and neuropsychological assessments is required, as well as complementary exams in specific cases. Among the complementary exams, genetic studies (chromosomes and CNVs), neuroimaging or neurophysiology studies stand out [51,53].
Despite significant economic and social costs, there are limited treatment options to improve symptoms associated with ASDs, including symptoms related to known diagnostic criteria and medical conditions that exacerbate the severity of presentation [22,54].
Currently, there are minimal evidence to support the benefit of most facials am ents. systematic reviews highlight the possibility of genetic heterogeneity, environmental, cognitive and social in ASD phenotype produces go sample studies of highly variables that reduce the effect size po tential of an intervention [55]. Other factors contributing to the difficulties in identifying effective treatments incluem small samples, using outcome measures that do not sound evenly ado mented and cultural differences in the perception of appropriate behavior 56. In addition, the study noted that the té 30% of cridence with ASD who participate in clinical trials may respond to placebo treatment, which could contribute to the reduction of the effect sizes of active intervention [56].
Generally, first-line treatments for children withASD include psychosocial treatment and educational interventions, with the aim of maximizing language acquisition, improving social and communicative skills and ending maladaptive behaviors [52]. The management of autistic individuals requires a multidisciplinary intervention, and the participation of well-trained psychologists or educators in functional behavioral analysis and behavior change techniques is essential. Due to the necessary structure and extensive resources, they become expensive, inaccessible to many children with ASD and their families [57].
However, there are few studies that prove the effectiveness of these interventions. Most of them present a fragile methodology, as they are generally studies with few participants and short-term follow-up [42].
The psychopharmacological treatment of children and adults with ASD is common in clinical practice. On average, about a third of individuals with ASD use some drug (psychotropic or vitamin) for the disorder or associated psychiatric and behavioral problems [58].
As the pathogenetic and pathological mechanisms are still unclear, there is no effective treatment drug for the eradication of autism that has been officially approved [59]. When used, pharmacological interventions generally target specific symptoms that accompany nuclear symptoms and severely the functioning of the individual, usually not allowing educational disrupt and behavioral interventions (eg aggression, irritability, agitation, self- destructive behavior), compulsive rituals and hyperactivity, etc.) [60]. Individuals with ASD are chronic patients, this vision therapy will extend for long periods, requiring professionals involved one finds monitoring, to have that exact scale of the problem. Resjumps also that the monitoring becomes necessary ary, since the agents commonly used in clinical practice belong to medication groups diverse, not being specific for target symptoms and also affect a broad spectrum of functions neurologicals and brain, not necessarily affected by autismo [55, 57]. Currently risperidone and aripiprazole are drugs approved by the US Food and Drug Administration (FDA) for the treatment of symptoms associated with ASD. These drugs have been shown to relief symptoms of irritability such as aggression and self-injury in adolescent autistic patients in several large clinical trials [60].
Precision medicine aims to provide personalized, evidence-based assessment and treatment for each patient and is therefore an advanced field of healthcare that is made up of each person’s unique clinical, genetic/genomic, biomarker and environmental information. This concept of precision has brought in the last decade a new perspective to the treatment of several psychiatric disorders including ASD [61,62].
To advance this new line of intervention there is a need for research with more precise information about ASD. Biobanks can be useful, as they are resources to generate clinical and scientific data for the analysis of medical conditions on a large scale [63,64]. These databases are collections of biomaterials with associated data. It is an essential tool for providing access to high-quality human biomaterials for research. These databases are collections of biomaterials with associated data. It is an essential tool for providing access to high-quality human biomaterials for research [66]. Some TEA-specific biobanks have been established in the
Associated with biobanks, studies with rodent models can contribute to elucidate the interaction between susceptible genes and risk factors, as they allow the evaluation and identification of molecular pathways and also have the ability to unify the neurophysiological mechanisms underlying each of the various etiologies that make up ASD [68]. Preclinical studies using animal models exhibiting autism-like features have demonstrated that directly altering gene expression using rAAV (recombinant adeno- associated virus derivative) delivered transgenes can reverse the behavioral phenotype, either through gene replacement or RNA knockdown [62].
The heterogeneity of the clinical manifestation and aetiology that is not understood is a marked characteristic of ASD. Although it has been a long time since its first description, the diagnosis is still based on observation of the behavior. Today, there is a need for knowledge and understanding of subgroups of individuals with ASD, this will be possible from the identification of comorbidities, genetic and physiological changes and trans - cultural factors that are imperative for the advancement of the research field. The definition of subgroups based on genetic, biological and elucidated markers from animal studies will allow objective and non-subjective identification based on responses. These genetic and biological signatures in the future may allow the knowledge of subgroups of individuals with ASD and thus the development of personalized and effective interventions.
NI: Manuscript writing and final revision.
IL: Coordinator; manuscript writing and final revision
The authors have no conflicts of interest to declare.
This work has not received any contribution, grant or scholarship.
This article is part of the doctoral thesis entitled “Identification of candidates for urinary biomarkers for Autism Spectrum Disorder”, presented to the Postgraduate Program in Sciences of the Disease Control Coordination of the São Paulo State Department of Health, to obtain the title of Doctor of Science. Author Dr. Nádia Isaac da Silva. Advisor Dro. Ivo Lebrun.
