Author(s): Aravind Reddy Kuchkuntla
Microangiopathic hemolytic anemias (MAHA) are secondary to damage of RBCs because of endothelial vascular damage of blood vessels leading to hemolysis. MAHAs are characterized by negative coombs test and are associated with several etiologies that include can be either hereditary complement or ADAMTS13 deficiency or sepsis or malignancy. Cancer associated MAHA (CA-MAHA) is a rare and is seen in patients with advanced metastatic disease. CA-MAHA has significant mortality rate and chemotherapy is the only therapeutic option, however overall survival is poor. Here we, present a rare case of CA-MAHA secondary to metastatic signet ring cell carcinoma with an unknown primary. In patients, when the cause of progressive MAHA is unknown, the possibility of cancer associated MAHA must be considered and a comprehensive work up for an underlying malignancy must be done.
Gastric cancer is the 5th most common malignancy in the world, and about 1 million cases are diagnosed every year around the world [1]. Gastric cancers are predominantly adenocarcinomas that account for 95% of the cases, followed by primary gastric lymphoma [1]. Based on the WHO classification, gastric cancers are classified into differentiated papillary, tubular, poorly cohesive and mucinous types based on the histopathological features. Recent trends in the United states have shown reducing incidence rates in the intestinal type due to effective H. Pylori treatment and an increasing incidence of poorly cohesive type. Among the poorly cohesive type of GCs, signet ring cell carcinoma phenotype is a sub-set with presence of signet ring cells and presence of poorly cohesive cells [2]. A signet ring is defined as a cell with ample cytoplasmic mucin which appears optically clear on haematoxylin and eosin (HE) staining and an eccentrically placed nucleus [3].
Primary SRC tumors arise from the GI tract, most common site is the stomach followed by colon, however case reports of signet cell carcinoma affecting lung, urinary bladder, skin, prostrate and breast have been reported [4]. Signet ring cell carcinoma (SRC) of the stomach affects 0.094% per 100,000 person years annually in the United States [5]. These are mostly undifferentiated to poorly differentiated pathological type and present in advanced stages with distant metastasis. Patients presenting with early stage SRCs have a good prognosis, but prognosis is poor in advanced cases [4]. The most common sites of metastasis are peritoneum, bone and less frequently liver and lung [6]. Apart from metastasis, SRCs have shown a tendency to be associated with various paraneoplastic syndromes [7].
Paraneoplastic syndromes are a group of heterogenous clinical manifestations secondary to physiological actions of tumor released mediators. It is established that tumors release hormones, hormone like peptides, growth factors and cytokines activating the host immune system against them eventually causing damage thorough cross reaction [7]. Commonly reported paraneoplastic manifestations of GCs are acanthosis nigricans, paraneoplastic acro-keratosis, paraneoplastic pemphigus, paraneoplastic dermatomyositis, sweets syndrome, SIADH, Cushing’s syndrome, carcinoid syndrome and acute hemolytic anemia [8]. In asymptomatic patients, presence of the above clinical features should raise suspicion for an underlying malignancy and needs to be evaluated. Here, we aim to present a patient who presented with low back pain, initial laboratory work showed direct antiglobulin negative hemolytic anemia and thrombocytopenia. On further work up, patient was diagnosed to have metastatic signet ring cell carcinoma with bone marrow infiltration causing bone marrow necrosis.
Cancer associated hemolytic anemia and thrombocytopenia is non-immune mediated hemolytic anemia due to thrombotic microangiopathy (TMA) from malignancy. CA-MAHA is characterized by negative coombs test, elevated LDH and bilirubin, low haptoglobin levels and thrombocytopenia with or without microthrombi formation or end organ damage [9]. Hemolysis inCA MAHA is abrupt onset that is often associated with severe thrombocytopenia needing transfusion of blood products. CAMAHA is commonly seen in solid organ tumors, most commonly gastric adenocarcinomas followed by breast cancer, prostrate and lung cancer. Majority of tumors are metastatic at the time of MAHA onset, prognosis is poor and is associated with high risk of mortality. It is essential to rule out TTP and HUS in such patients as plasmapheresis is ineffective in management of CA-MAHA. Treatment of the underlying malignancy has shown to revert the hematological changes of MAHA, however clinical response rates are grim [10].
60-year-old male with past medical history of diabetes type 2, hypertension, hyperlipidemia presented with low back pain and epistaxis for 1 week prior to admission. Patient was in usual state of health until one month prior to presentation and reported that he started developing low back pain, that was worsening. Patient described the back pain to be 6/10, predominantly in the sacral region with no numbness, tingling, lower extremity weakness or loss of bladder or bowel control. Patient denied any other complaints, recent infection, however he reported that he has lost 15 pounds in the last 2 to 3 months. Patient denied any family history of malignancy, surgical history of any significant malignancy in the past, denied smoking, drugs, allergies. On physical examination was unremarkable.
Labs on presentation: hemoglobin: 6.9, platelet: 9k, MCV: 101.5, wbc: 6.9, differential 54.4% neutrophils, 35.3% lymphocytes, nucleated RBCs high: 18, RBC morphology: notable for no schistocytes and platelets had normal morphology. Prothrombin time: 13sec, INR: 1.1, aPTT: 30 CMP: total protein: 6.8, renal function was normal (Cr: 0.91; BUN: 23), liver function showed elevated alkaline phosphatase 948 with elevated total bilirubin: 3.9 mg/dl, GGT: 16. LDH elevated 1448, haptoglobin: <30, fibrinogen: 193. CT abdomen pelvis showed normal liver, spleen, kidneys and prostrate with enlarged lymph nodes within the retroperitoneum most pronounced appearance in upper pelvis with largest node measuring 2.4 cm x 1.5 cm. Other significant finding on CT was abnormal density pattern with multiple areas of increased density patterns suspicious for osteoblastic metastatic involvement within pelvic bones and spine. MRI of the abdomen confirmed presence of malignant appearing lymphadenopathy in the retroperitoneum and in mesenteric root along with presence of heterogenous signal within marrow of several thoracic and lumbar vertebral bodies.
CT chest with contrast showed right paratracheal lymph measuring 17 mm x 10 mm, 11 mm left pre-vascular lymph node and extensive hilar lymphadenopathy with no lung masses or axillary lymphadenopathy. Nuclear bone scan showed numerous foci of increased activity in bilateral ribs, cervical, lumbar, thoracic, lumbar spine, bilateral sacrum, left iliac bone, bilateral scapula, and proximal femoral neck. Given the presentation with hemolytic anemia and metastasis with unknown origin patient was admitted for further work up.
Initial work up of tumor markers was negative with normal PSA: 0.5 ng/ml, CA 19-9: 10.6 U/ml, AFP: 2.5 ng/ml, CA 125: 49 U/ml and hCG tumor marker 1.4 mIU/ml except for a mildly elevated CEA: 27.7 ng/ml. Given the anemia patient underwent an upper GI endoscopy and colonoscopy that was negative for bleeding, but random biopsies were taken and were negative for any neoplastic process. IR guided biopsy was taken from the right pelvic lymph node and peri-aortic lymph node were done that showed no definitive evidence of malignancy. Lymph nodes were reported as reactive nodes with focal histiocytic inflammation with no malignant features. Bladder cancer assay was negative with a normal signal pattern was observed for CEN 3, CEN 7, CEN 17, and the 9p21 probes. Serum protein electrophoresis showed albumin 3g/dl, alpha 1 0.5g/dl, alpha 2: 0.4g/dl, beta: 0.6g/dl, gamma globulin: 9.g/dl, overall interpretation negative for a M spike and low alpha 2 suggestive of hemolysis. Peripheral flow cytometry showed no T cell or B cell immunophenotypic abnormalities or presence of blasts. Karyotyping showed normal male phenotype with no abnormal clones.
Mediastinal and right para-tracheal biopsy showed malignancy with signet ring morphology, immunostaining positive for BerEP4, MOC31, WSK, CK 7 +/- and CDX2 and was negative for PSA, GATA3, TTF1, and PAX8 suggestive towards a GI tract malignancy (Figure1). Immunostaining for Her2 was equivocal likely due to mucin. Bone marrow biopsy showed a hypercellular bone marrow with extensive coagulative necrosis, focal viable trilineage maturing hematopoiesis, mild erythroid dyspoiesis and no blasts. Extensive coagulative necrosis suggested towards a thrombotic event. Rare foci of atypical epithelial cells with signet ring morphology were noted that showed positivity on immunostaining for MOC31, Berep4, and WSK. CK 20, CK 7, CDX2 were negative (Figure 2 and Figure 3). Diagnosis of metastatic signet ring cancer with an unknown primary was made with the results of bone marrow and lymph node biopsy
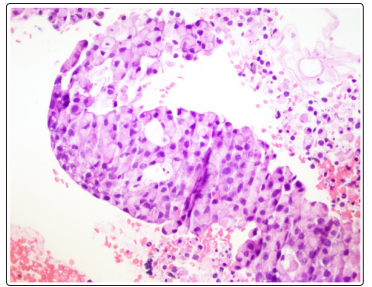
Figure 1: Lymph Node with Signet Ring Cell Morphology
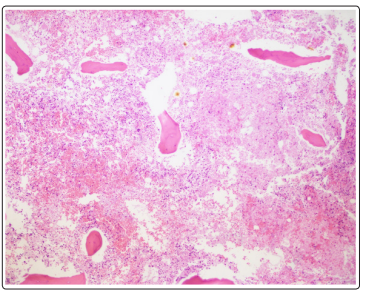
Figure 2: Necrotic Bone Marrow
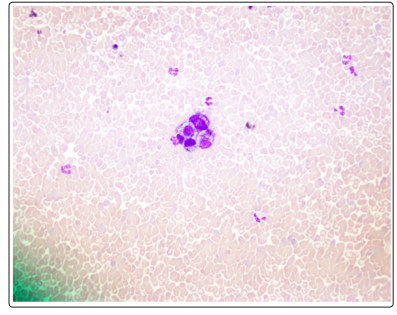
Figure 3: Bone Marrow Aspirate Smears with Rare Tumor Cell Nests
Initial differential for anemia and thrombocytopenia was hemolytic process versus malignancy as initial labs showed macrocytic anemia with elevated reticulocyte count, negative DAT associated with low haptoglobin and elevated LDH and bilirubin. ITP was ruled out as patient did not have any recent infections or exposure to triggers, inherited defects unlikely as there was no family history and lab work in the past was normal, mechanical hemolysis ruled out as patient had no mechanical heart valves or severe aortic stenosis, spleen was normal ruling out splenic sequestration. Other work up of auto-immune antibodies, complement, hiv, acute hepatitis panel, flow for paroxysmal nocturnal hemoglobinuria, covid IgG antibody were negative. Glucose 6-phosphate dehydrogenase was mildly elevated 25 U/g Hb, ADAMTS13 activity was normal at 64%. HUS, TTP, DIC were ruled out as patient had no neurological or renal failure, no coagulopathy and normal ADAMTS13 activity, making diagnosis of thrombocytopenia secondary to metastatic signet ring cell carcinoma likely.
Patient was initially admitted to the hospital for evaluation of hemolytic anemia and a possible malignancy initial imaging was suggestive of generalized lymphadenopathy. During the initial course of admission patient was started on prednisone 80 mg daily and was managed conservatively with FFP transfusion and blood transfusions. Plasmapheresis was not considered as patient did not evidence of HUS/TTP. Patient’s hemoglobin remained between 6.5 g/dL 8 g/dL despite receiving several PRBC transfusions. Patient remained severely thrombocytopenic and did not show any response to steroids. During admission patient developed bicytopenia and leukoerythroblastic picture with elevated nucleated RBCs, leukopenia with very few metamyelocytes suggestive of bone marrow infiltration for malignancy
After the bone marrow biopsy was resulted it was determined that the patient was a candidate for chemotherapy however patient’s clinical condition deteriorated as patient developed acute hypoxic respiratory failure and eventually became septic with positive blood cultures for Clostridium perfringens. Patient was also diagnosed possible abdominal viscus perforation. Due to the worsening clinical condition of the patient with active infection and a possible perforation, decision was made not to give any chemotherapy even at the reduced dose as the prognosis was poor with high risk of mortality. Decision was made to discuss comfort care with the family and patient was discharged to an inpatient hospice.
Cancer associated hemolytic anemia and thrombocytopenia is nonimmune mediated hemolytic anemia characterized by a negative coombs test. Hemolytic anemia presents with fragmented RBCs or schistocytes on peripheral smear, and biochemical findings of hemolysis with elevated lactate dehydrogenase (LDH) and indirect bilirubin and low haptoglobin. Constellation of these findings is suggestive of a thrombotic microangiopathy (TMA) that is characterized by non-immune hemolytic anemia with end organ damage and formation of microthrombi.
Primary TMAs are either hereditary disorders such as ADAMTS13 deficiency or mutations in complement/coagulation pathways or acquired disorders such as thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS) or drug mediated immune reaction. Secondary TMAs due to direct effect of systemic infection, cancer or autoimmune disease and treatment of the cause leads to resolution of TMA [11]. Cancer associated hemolytic anemia (CA-MAHA), is therefore a secondary TMA and treating the cancer is therapeutic. Plasmapheresis is generally not indicated in such patients as its unclear that patients with metastatic disease have low levels of von Willebrand factor-cleaving protease [12].
Pathophysiology of MAHA in cancer patients is unclear, however several hypothesis and theories have been put forward. Initial pathogenesis was thought to be direct injury of RBCs due to mechanical damage or as a result of RBC injury as a result of contact with tissue thromboplastin released from tumor cells via the activation of factor X by pro-coagulant A secreted from mucinous adenocarcinomas [13]. It was also put forward that MAHA represented a spectrum of hematologic abnormalities associated with neoplasia, that ranged from thrombophlebitis, nonbacterial endocarditis, and DIC with MAHA possibly occurring in a setting of sub-clinical DIC with presence of few fibrin thrombi and no evident coagulopathy [14]. In a case series, findings suggestive of micro tumor emboli, intimal wall proliferation and pulmonary thrombotic microangiopathy were reported supporting the direct mechanical damage to RBCs in small vessels [9].
Another hypothesis states that injury to the endothelial cells in the bone marrow by direct tumor invasion can lead to accumulation of large VWF, reducing ADAMST13 thereby causing platelet aggregation [15]. Patients with CA MAHA present with acute onset anemia due to hemolysis; initial laboratory work up should be focused on ruling out other etiologies of non-immune mediated hemolytic anemia. Work up in our patient was negative for complement mediated hemolytic anemia, cold agglutin mediated hemolytic anemia, infectious causes, autoimmune causes, lymphoproliferative malignancies, DIC, ITP, mechanical damage secondary to prosthesis and splenic sequestration. Patients with CA MAHA can present with or without neurological symptoms or renal failure suggestive of wide spectrum of presentations.
Patients with neurological symptoms can be coined to be TTP predominant CA MAHA, patients with renal failure fall under HUS predominant CA MAHA and with none of the above are patients with MAHA alone like our patient. Significant discrepancies exist in description of CA MAHA presentation and uniformity is lacking in literature due to the rarity of diagnosis. Despite aggressive therapy, prognosis in patients with MAHA is extremely poor with an average survival of 21 days in a case series that evaluated 55patients with CA-MAHA [14]. In another case series, 46% of patients died within one of diagnosis with or without treatment [9]. Only effective therapy is chemotherapy, patients who respond to therapy show resolution of hematological changes and hemolysis, reduced lymph node size and shrinking of bone lesions. Response rates are poor and as 90% of patients with MAHA have distant metastatic disease at presentation overall survival is exceptionally low [16].
SRCC frequently affects the bone and patients with bone marrow involvement present with characteristic leucoerythroblastic picture on peripheral smear [17]. Another rare presentation is myelophthisis and bone marrow necrosis. Bone marrow necrosis mostly occurs in hematological malignancies, however it’s been reported in SRCC with MAHA where in there is necrosis of the medullary stroma and myeloid tissue with preserved bone tissue [18,19]. Presence of extensive coagulative necrosis in our patient supports the hypothesis of endothelial damage in the bone marrow could be the inciting event for progression to MAHA.
In a case series, 24 patients with MAHA secondary to gastric cancer who underwent treatment with chemotherapy were reviewed, 9 out of 24 patients had signet ring cell carcinoma. Majority of these patients with signet ring cell carcinoma were treated with different cytotoxic chemotherapy agents such as 5FU, Adriamycin, mitomycin C, etoposide, leucovorin, 5FU, cisplatin, 5FU, vincristine. Overall, survival was limited from weeks to couple of months however one patient had a reported survival of 19 months. In this specific patient complete hematologic resolution was attained with use of cisplatin and 5FU however the patient relapsed was treated successfully with docetaxel [20].
In a recent case series of 10 signet cell carcinoma with MAHA as an initial presentation, 80% of patients presented with either fatigue or bone pain, and an average hemoglobin on presentation in reported patients was 6g/dL, platelets ranged between 30k to 86k [21]. All the patients presented had bone metastasis, 50% had metastasis to bone marrow and 50% had multiple bone metastasis. Underlying cancer in 6 patients was gastric or GI tract, 3 patients had an unknown origin, and one patient had an ovarian cancer. 90% of the patients did not survive beyond 4 weeks. Since the publication of this case series, signet ring cell carcinomas of intrahepatic bile duct, breast and jejunum have been reported with MAHA and poor outcomes on chemotherapy [22-28].
CR MAHA is an exceedingly rare presentation in patients with advanced metastatic disease. Given the mortality associated with it, pathophysiology largely remains unclear and the therapeutic options are limited as well. Future research must be done investigate the pathophysiological mechanisms of MAHA, standard guideline framework needs to be established to bring a uniform approach to come up with a streamlined approach.
Conflict of Interest: None for all authors
Patient consented for the publication of case report.
