Author(s): Mohamed Nagar S*, F A El-Sayed, W M Morsy, A H Mady and A M Mohamed
Detailed studies of uranium heap leaching from different uranium mineralization resources situated in Eastern Desert of Egypt were investigated using sulfuric acid via batch experiments, followed by using the obtained optimum condition on bench scale leaching tests using small column. The previous optimum parameters were implemented on another large scale column in order to make more condition control and evaluate the time and reagents needed while transferring from small to large scale. The obtained data shows that leaching efficiency of GII mineralization attained about 78.3% with consumed acid amount about 34 kg/ton in a 44 days as leaching time, on the other hand, leaching efficiency of uranium from El-Missikat mineralization higher than GII mineralization and attained about 86.6% and the consumed as lower than G-II about 28kg/ton in a 40 days leaching time. Kinetics reaction models of column tests have been investigated to optimize the column leaching behavior. Based on the leaching results of the two mineralized occurrences, the rate of the uranium dissolution is controlled by both the chemical reaction and the diffusion reaction but diffusion reaction control was more predominate than a chemical reaction.
Heap leaching is an industrial mining process used to extract precious metals, copper, uranium, and other compounds from its low grade bearing ores or mineralization’s using a series of chemical reactions. In uranium heap leach mining, from its mineralization places on heap pad, followed by adds the chemicals reagents via drip irrigation systems to the ore [1].
The main heap leach mining works suggested for large volumes of low grade ores as, it reduce the coast of metallurgical treatment of the used ore. The significantly reduced processing costs are offset by the reduced yield of usually approximately 60-70%.Also the main advantage of the heap process is the amount of overall environmental impact caused by heap leaching is often lower than more traditional techniques [2].
Progress of heap leaching studies by using column leaching methods have the advantages of allowing observers to study longer term chemical interactions between solid samples and leachates, to note changes in the permeability of solid samples with time, and to evaluate how chemical reactions may change once more soluble compounds are flushed out of the solids [3] .
In Egypt Several promising uranium occurrences have been discovered in the Eastern Desert as, Gable Gattar , El-Missikat, Abu Rushed and El-Sela areas. Gabal Gattar are allies in the northern part of the Eastern Desert at the intersection of coordinate 27°06’N and 33°16’E at a distance of 95Km from Hurgada City, at the Red Sea Coast. The mineralization mainly associated with granite as in G-II occurrence and with Hammamate sedimentary rocks as in case of G-V occurrence. According to Mahmoud, petrographical examination of GII fresh granite has revealed that it is mainly composed of orthoclase perthite (with subordinate microcline perthite), quartz and plagioclase beside minor amounts of biotite and muscovite [4]. On the other hand, the accessory minerals are represented by zircon, fluorite, apatite and sphene [4].
El-Missikat uranium prospect area lies at about 3 km, midway between Safaga, on the red Sea coast and Qena in the Nile Valley. It is roughly bound by longitudes 33°15`-33°28` E and latitudes 26°24` - 26°30` N, where the mineralogical studies revealed the presence of uranium minerals such as uranophane, uraninite, soddyite and renadite [5]. The main accessory minerals are sulfides, magnetite, zircon, apatite, fluorite, titanite, monazite, xenotime, uranothorite, rutile and uraninite. Hematite, epidote, muscovite and chlorite are present as secondary minerals [6,7].
Several works have been achieved to leach U and associated valuble elements from G-Gattar prospect. Leaching of uranium and molybdenum from G-Gattar mineralization using acid and alkaline agitation leaching was studied. Acid leaching has indicated that complete leaching of U/MO by using 50g/l H2 SO4 and solid/ liquid ratio of 1/2 at room temperature for an agitation time of 12 hr. About 95.1% of uranium leaching efficiency was obtained at 60 °C for 8 hr by using 50g/l Na2 CO3 or NaHCO3 in case of alkaline leaching [8-10].
Uranium percolation leaching from both Gattar-II and Gattar-V mineralized samples were also studied. From the obtained results, the particle size has a significant impact on the leaching efficiency. Regarding to GII mineralization, the leaching efficiency of -10 mm sample is 76.9%, but the leaching efficiency of -40 mm sample is 47.4% [11].
The leaching studies achieved on El-Missikat attained the efficiency reached to 91% after 8 hr of agitation [12]. In the same path, the contained REE leaching is studied during uranium recovery, which achieved leaching efficiency of about 95 %. [13,14]. Agitation and column percolation leaching techniques applied upon the uranium rich mineralization (El-Missikat) showed that these techniques succeeded in providing considerable results [15].
The present work concerned with the amenability of heap leaching application via study the optimum conditions required for uranium dissolution from the two previous uranium occurrences. Kinetics reaction models of column tests have been investigated to optimize the column leaching behavior.
Two representative composite samples were used to carry out this study one from Gabal Gattar and the other from El Missikat occurrences. The chemical composition for both major and trace elements are shown in Table 1.
Table 1: Chemical composition of GII and El-Missicat representative sample| Oxide | GII | El-Missikat | Trace Elements | GII | El-Messicat |
|---|---|---|---|---|---|
| % | % | Ppm | Ppm | ||
| SiO2 | 75.30 | 87.97 | Mo | 49 | 4 |
| TiO2 | 0.28 | 0.11 | Co | 5 | 5 |
| Al2O3 | 10.30 | 3.4 | Zn | 220 | 400 |
| Fe2O3 | 2.10 | 4.4 | Ba | 71 | 200 |
| FeO | 0.53 | 0.58 | U | 1300 | 1850 |
| MnO | 0.02 | 0.1 | REEs | 85 | 130 |
| MgO | 0.50 | 1.16 | Zr | 30 | 200 |
| CaO | 1.69 | 0.55 | Cu | 16 | 100 |
| Na2O | 3.50 | 0.078 | Th | 29 | 20.5 |
| K2O | 3.40 | 0.068 | Nb | 88 | 87.5 |
| P2O5 | 0.50 | 0.75 | Sr | <2 | 201 |
| L.O.I | 1.60 | 1.7 | Cr | 4 | Nil |
| Total | 99.72 | 100.7 | Ni | 5 | 80 |
| Pb | 87 | 417 |
From the table (1) it is obvious that, the two analyzed samples nearly similar in major oxides, except that the El-missikate show higher in silica content relative to Gattar sample and also have more economic elements such as REEs, Ba, Cu and Sr , relative to GII
All acid leaching testes were carried out using distilled water and concentrated sulfuric acid H2SO4 (Merk) as the lixivant solution and A.R grade of other chemical reagents. The hydrogen ion concentration (pH) of the different solutions was measured accurately using the pH- meter model (HAANA pH-mV-temp).
This type of analysis was performed upon 1.0 Kg as representative sample from the studied occurrences where the two samples were subjected to both crushing and sieving along with a range of sizes from - 1.25 to - 0.25 mm. After that, all fractions of grain size were analyzed for uranium.
To study and determine the optimizing factors affecting the uranium dissolution using acid agitation, a series of agitation leaching experiments were performed after selecting the appropriate sample weight, ground to appropriate size and was mixed well with a suitable volume of different sulfuric acid concentrations. The studied factors are, grain size, uranium distribution, agitation time, and suitable sulphuric acid concentration.

Leaching experiments were conducted using PVC columns (5 cm x100 cm high).To avoid the “side wall effect”, the inner wall was polished with sandpaper in advance to increase the roughness. The top of the leaching column was opened; the bottom was reserved for the outlet of the duct to collect the leachate and was covered with 5cm layer of the 5-mm-thick quartz sand particles.
Uranium was analyzed in the corresponding low concentration of aqueous phases using ArsenazoIII reagent under different conditions [16]. In high concentration (? 10ppm) uranium was determined in the pregnant solution and the crude uranium concentrate using the oxidimetric titration procedure with a standard solution of NH4 VO3 till the appearance of a purplish red color represents the end point [17].
Chemical composition of GII and El-Missikat uranium occurrence From table 1, iron percent is relativity high about 2.1 and 4.4% Fe2 O3 and 0.53 and 0.58 % as FeO in GII and El-Missikat respectively, and therefore controlled leaching conditions must be applied to minimize iron dissolution in order not to interfere with uranium during its latter recovery. So that, sulfuric acid is excellent reagent used to dissolve uranium. Since most uranium minerals can be leached only when they have been oxidized, oxidizing conditions are maintained by ferric ions, which are generated by the oxidation of the ferrous ions present in solution [18]. Oxidation effect could be achieved by pyrolusite (MnO) which exist also as a major oxide in the two operated samples (0.02 and 0.01%) respectively
On the other hand, El-Missikat sample contains some deleterious elements such as Ti, Mo, Th, Pb and Zr at relatively higher to trace level than GII ore sample, and might cause either chemical poisoning and/or physical fouling in case of uranium recovery by ion exchange resin. So that, the leaching solution concentration should be lower than 100mg/l to avoid their dissolution. As Fe+3/ Fe+2 ratios greater than 2, no need for adding oxidant to achieve uranium dissolution reaction [19,20].
Presence of silicate and iron oxide as gangue minerals lead to consume sulfuric acid during initial reactions. K-feldspar, Nafeldspar, Ca-plagioclase and Biotite breakdown to Ortho-silicic acid, H4SiO4, and various metal cations such as Na+ , K+ , Ca2+, Fe2+, and Fe3+. The ferric iron concentration in leach liquor is controlled by adjusting the redox potential by the addition of oxidant. For typical leaching conditions the relationship between the redox potential and the iron concentrations is given by the Nernst equation [21].
Ec = 397+0.19847 T log ([Fe3+]/[Fe2+] ) ?????.. (1)
Where Ec is the solution potential relative to the saturated calomel electrode at 35°C (mV), is the molar concentration and T is the temperature (K). Equation (1) shows that at an oxidation potential of 400 mV about 50% of the iron is in the ferric state, while at 500mV only 2% remains as ferrous ion [20].
Heap leaching technique produce a huge amounts of pregnant leach solution which get difficulty to pretreatment before loading, so that require low H2 SO4 concentrate to keep the resulting leacheat pH not exceed 1.5. In addition to decrease the solubility of other associated elements [22].
Uranium distribution was investigated in the study samples for each grain size faction, Table 2 for determining the most suitable size to achieve the aim of the study.
Table 2: Granulometric analysis and uranium distribution in the sample| Size (mm) | GII | El_Missikat | ||||
|---|---|---|---|---|---|---|
| Fraction weight, (g.) | Size distribution wt.% | Assay of Uranium, ppm | Fraction weight, (g.) | Size distribution wt.% | Assay of Uranium, ppm | |
| +1.25 | 140 | 14.0 | 70 | 140 | 14.0 | 41 |
| -1.25 to +0.5 | 437 | 43.7 | 122 | 437 | 43.7 | 33 |
| -0.5 to +0.25 | 180 | 18.0 | 149 | 180 | 18.0 | 9.4 |
| -0.25 | 245 | 24.5 | 350 | 245 | 24.5 | 17 |
| Total | 1002 | 100.2 | 172 | 1002 | 100.2 | 100.4 |
A series of experiments were designed to study in detailed the effects of several variables on uranium leaching from gattar and El Missikate technological samples such as acid consumption, solid\liquid ratio, grain size, contact time, and PLS pH.
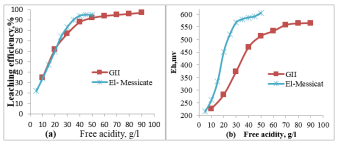
Figure 1: Effect of (a) free acidity (b) Eh on leaching of uranium
From the obtained data shown in Figure 1, it is clear that, the uranium leaching efficiency increases as periodically from 35 to 92 %, as the acid concentration of leach solution increases from 10 to 50 g/l, then tends to be stable after that. On other hand, behavior shows considerably increase in uranium leaching efficiency (17 to 94) with lower increase in acidity (5 to 40g/l). From these data, it is concluded that the iron content in the ore sample play important role in the leaching efficiency, which accelerate the uranium dissolution with lower acid need. Figure 1b can show more data which verify the above conclusion. Since, the redox potential increases in leaching process more rapid in El-Missikat than GII ore sample. From these data, it is concluded that an economically 40 and 50g/l acid concentration is the best one for El-Missikat and GII respectively
Leaching experiments were performed over a range from 30 up to 200 min. Other variables were fixed at the leaching conditions of 40 g/l sulfuric acid, 1/2 solid /liquid ratio, at 25 °C temperature and grain size of -0.25 mm. The obtained data show that uranium leaching efficiencies increase with increasing contact time achieving its maximum after 120min. Increasing time over 120 min was found to be ineffective as shown in Figure 2.
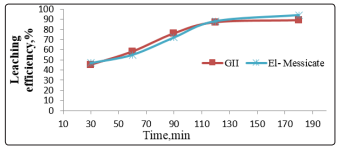
Figure 2: Effect of contact time on leaching of uranium
As in shown in Figure 3, the effect of solid/liquid ratio on the dissolution of uranium was studied using 1:1, 1:2, 1:3,1:4 and 1:5 solid/liquid ratios, while the other testing parameters comprised at 40g/l sulfuric acid conc., 180 min. agitation time, 25°C temperature, and 150 r.p.m. agitation speed.
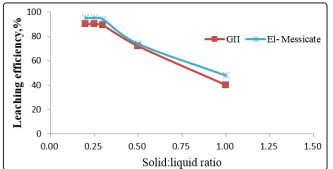
Figure 3: Effect of solid: liquid ratio on leaching of uranium
The amount of solid was kept constant, and the liquid volume was changed to obtain the desired solid/liquid ratios. From these data, it was found that beyond 1/3 S/L ratio, only slight steady increase in the leaching efficiencies of uranium has been achieved. Accordingly, a solid ratio of 1/3 would be considered as optimal ratio at which the uranium leaching efficiency of attained 89 and 92 % for El-Missikat and GII, respectively.
Effect of Grain sizeThe effect of the grain size on the uranium leaching efficiency is achieved by studying the grain size of mineralized samples ranged from + 1.25 to - 0.25 mm. Other leaching conditions were fixed at 40 g/l acid concentration, 1/3 solid/liquid ratio for 120min agitation time at room temperature. The results obtained are shown in Figure 4, as shown uranium leaching efficiency has increased from 48 to 89% and 52 to94% for El-Missicat and GII, respectively with decreasing the crashed size from +1.25 to -0.25 mm. This can be explained by the fact that by decreasing the grain size, the surface area exposed to the reaction increases and hence the percentage of extraction also increases.
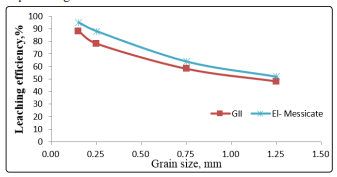
Figure 4: Effect of grain size of ore sample on leaching of uranium
Column leaching (5.0cm diameter and 100cm high) experimental was performed to study the effect of the following parameters on uranium dissolution and acid consumption: ore particle size, application rate, Iixiviant acid concentration, ore height, and ore grade. The experiment was expected to generate a reduction in acid consumption without affecting uranium dissolution.
The effect of the H2 SO4 concentration was studied from 20 to 50g/l on the uranium leaching efficiency on column during 25day at room temperature with -0.25mm.The derived plotted figures 5 (a,b) shows that the same result that obtained on the batch experiment. Since, the uranium increase with free acidity increase and tend to be stable as free acidity increase from 40 to 50g/l.
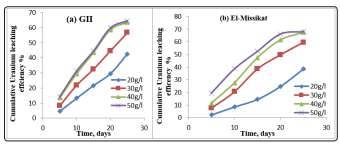
Figure 5: Effect of free acidity on uranium leaching during column leaching of GII and El-Missicat
In this type of leaching the columns were packed with differing the particle size from +0.25 to -0.25 with fixing the other conditions, 30g/l H2 SO4 and 1/3 solid liquid ratio were achieved during 25 day. Their plotted Figures 6 (a,b) shows the directly increase in uranium leaching with decrease in the particle size which owing to the increase in the particle surface area at which the reaction takes place.
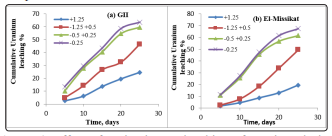
Figure 6: Effect of grain size on leaching of uranium during column leaching of GII and El-Missicat
As shown in pervious figures there are evident that the mechanism of leaching of the short-time (batch experiment) is different from that of the long-time (column experiment). Since the slopes for the curves at these periods (120min for batch and 25 day for column) are noticeably different.
The dissolution kinetics of uranium was studied to understand the rate-controlling step and to optimize the leaching process. As uranium leaching is heterogeneous reaction including more than one phase, specifically fluid and solid phase, shrinking core model (SCM) for spherical particles of unchanging size can be used to study the kinetics. With respect to the study of the liquid-solid reaction kinetics, many different mathematical models of kinetic reactions, such as the unreacted core shrinking model, and the particle model, have been proposed. One of the most important models is the unreacted core model, which has been successfully and extensively used [23,24].
Based on the research results of Z. Ekinci, the uranium ore leaching fractal dynamics can be studied with the unreacted core model and the reaction is shown as follows [25].
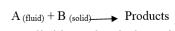
If the action is controlled by a chemical reaction, the reaction kinetics is given as follows:
1-(1-X)1/3=K1t (3.1)
Where X is the ratio of the accumulated amount of the leached uranium to the total content of uranium in the ores, and K is the uranium dissolution rate (g d-1).
If the action is controlled by diffusion through a metal-ore surface, the reaction kinetics equation can be written as follows:
1-3(1-X)2/3+2(1-X)=K2t (3.2)
Applying a regression analysis to the tested data by using these equations, it is found that the rate of the uranium metal dissolution is controlled by the chemical reaction and the diffusion reaction. Based on the leaching results of two mineralized samples, the integrated rate values of the leaching for every tested sample are described by equations (3.1) and (3.2) and shown in derived figures.
Figures 7a (GII and El-Missikat) derived from pervious column tested results, shows that 30g/l H2 SO4 is the excellent concentration for applying the column leaching in two mineralized samples on basis of chemical reaction model.
On basing the diffusion model as shown in Figures 7b (GII and El-Missikat), the situation differ than in chemical model. Since, the 40 and 50g/l are preferred concentration for GII and El-Missikat mineralized samples respectively. Thus, the speed of the uranium dissolution reaction increases, and the chemical reaction control occurs much earlier than the diffusion reaction control in the leaching experiment.
In order to obtain the reaction order for the total H2SO4 concentration, log-log plots of the rate constants versus the total H2SO4 concentration are plotted and given in Figure 8.The reaction order was determined to be about 0.920 and 0.983 for GII and ElMissikat mineralized samples respectively, which indicate strong dependence of the rate on H2SO4 concentration.
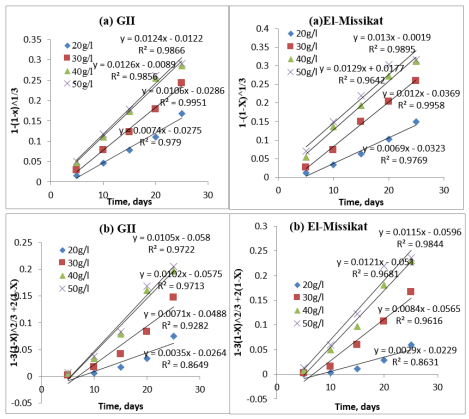
Figure 7: The kinetic curve of uranium leaching with different H2SO4 concentrationbased on different models. (a) Chemical reaction contro (b) Diffusion reaction control
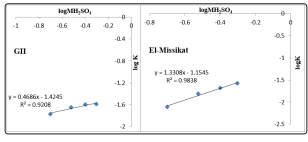
Figure 8: log-log plot of the rate constant versus H2SO4 concentration
Figures 9a, shows the integrated rate values of the leaching kinetic for the mineralized samples with different fractal dimension based on the chemical reaction control model. The reaction rate reaches a maximum value after 20day, where showed excellent result at (-0.5 to +0.25) and -0.25mm in case of GII and El Missikat mineralized samples respectively. Since give the higher slops 0.986 for two sizes of two mineralized samples.
Figures 9b, which based on the surface diffusion reaction control model shows lower slopes of dissolution rate curve than other of the chemical reaction control model. This can be attributed to the dependence of uranium dissolution rate on surface diffusion reaction control than chemical one. However, as the chemical reaction goes on, the heat of the chemical reaction has been accumulated and the movement of the molecular collision has been aggravated, then the leaching solution diffuses into the nucleus of the ores gradually; afterwards, the diffusion reaction rate also increases gradually
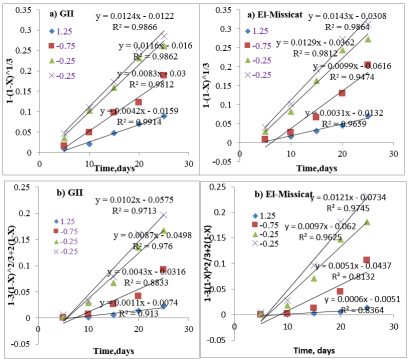
Figure 9: The kinetic curve of uranium leaching with different particle size based on different models (a) Chemical reaction control; (b) diffusion reaction control
The apparent rate constant was determined and plotted versus the initial average particle size and the results are shown in Figure 10. The linear relationship between the rate constant K, and the particle size indicates that the ash layer diffusion reaction on the particle surface is the rate-limiting step of the dissolution process. As shown in Figure 10, GII ore samples gives higher slop than El-Missikat one, which indicate that GII ore samples gives higher dissolution rate with particle size decrease than El-Missikat one.

Figure 10: Plot of the Uranium dissolution rate constant versus the average of the particle size
The amenability of heap leaching of uranium from low grad ores of GII and EL-Missikat were investigated via batch and column tests. Kinetics models were applied on column leaching tests to understand the rate-controlling step and to optimize the leaching process. A diffusion reaction control was more predominate than a chemical reaction. Finally, the leaching behavior was analyzed and studied, besides verified by kinetic models in order to prepare of the pilot scale.
