Author(s): Thais Manfrinato Miola*, Susana da Rocha Dias, Magna Aparecida Veridiano Matayoshi and Rafaela de Araujo Campos
Background: Breast cancer is the most common cancer among women worldwide. This case report aimed to evaluate the effect of specialized oral nutritional supplements (ONS) in the prevention and treatment of radiodermatitis in patients undergoing radiotherapy (RT) for breast cancer.
Methods: Case report of three patients during radiotherapy treatment, one using specialized supplementation with a preventive objective and another to treat radiodermatitis, the third patient did not use any supplement, being the control.
Results: Patients who used ONS showed beneficial results regarding the appearance of radiodermatitis and evolution of its degree when compared to the control patient. Patient 1 had radiodermatitis only at the end of RT with grade I toxicity. Patient 2 had radiodermatitis in the middle of treatment, where ONS started, with grade I toxicity and the lesion did not progress until the end of treatment. On the other hand, patient 3 had grade I radiodermatitis in the 10th session, evolving to grade II in the 23rd session.
Conclusions: It was concluded that the use of specialized ONS helps in the healing process of radiodermatitis in patients undergoing RT in the breast region, in addition to maintaining/improving body composition.
Breast cancer is the most common cancer among women worldwide, impacting 2.1 million each year. In Brazil, 66,280 new cases are estimated for the triennium 2020-2022 [1].Among the therapeutic options for the treatment of breast cancer is radiotherapy (RT), which can be performed as adjuvant therapy to reduce locoregional recurrence, or as neoadjuvant therapy, aimed at reducing tumor volume before the main treatment [2].
Despite advances in radiation techniques, adverse effects, especially on the skin, called radiodermatitis, are observed during and after RT treatment. Radiodermatitis can vary from grade I to IV according to the severity of the lesion, which can range from mild erythema and itching, to dry or moist desquamation, which can cause tissue necrosis. It is estimated that 95% of Patients treated with RT develop some form of skin reaction [3].
The presence of radiodermatitis directly affects the patient’s quality of life. Among the effects presented, we found local hypersensitivity, itching, pain from exposure of nerve endings, loss of the skin’s protective barrier, leaving the body exposed to opportunistic infections, in addition to changes in body image, self-image and self-esteem leading to social isolation [3]. In a study of 100 women with breast cancer undergoing RT treatment, it was observed that there was a worsening in the quality of life of these women according to the degree of radiodermatitis, considering work/school, daily activities, symptoms, and feelings [4].
Some factors can enhance the degree of radiodermatitis, such as age, nutritional status, tumor location, irradiated volume, among others [5]. Adequate nutritional status provides energy, Protein substrates and micronutrients that support the healing process. Protein depletion prolongs the inflammatory phase and impairs fibroplasia, as there is a decrease in fibroblast proliferation, angiogenesis and collagen production [6]. The use of specific nutrients helps in wound healing and the literature is wide regarding the use of these nutrients in pressure injuries (LPP), but little is known about possible benefits in the prevention or treatment of radiodermatitis.
The main nutrients in formulas used in the healing process are arginine, glutamine and amino acids, which play a role in biochemical pathways responsible for mediating collagen synthesis and immune response [7]. Arginine, despite comprising a small amount of the collagen mole-cule, plays an important rolein protein synthesis, exerts immunostimulating functions and acts as a proline precursor, which together with its metabolite hydroxyproline constitute 1/3 of the collagen-forming amino acids. Glutamine is the most abundant free amino acid in the body, being classified as a conditionally essential amino acid. In the healing process, glutamine has been shown to be important because it is related to collagen synthesis and proliferation of inflammatory cells [8].
Micronutrients, Zinc, Selenium and Vitamins A, E, and C, are essential for the healing process being fundamental, as they act as cofactors in all phases of collagen synthesis [7].
Cereda et al (2015) observed beneficial results with the use of these specific nutrients [9]. Of these specific nutrients in 200 patients from a long-term care facility for 8 weeks. Participants were divided into 2 groups: a standard oral diet + oral nutritional supplement (ONS) group - control group - and a standard oral diet + ONS enriched with arginine, zinc and antioxidants - intervention group. The result was greater reduction in the area of the lesion, (n=101), mean reduction of 60.9% [95%CI, 54.3% to 67.5%], compared with the control formula (n=99 ) (45.2 % [CI, 38.4% to 52.0%]) (adjusted mean difference, 18.7% [CI, 5.7% to 31.8%]; P = 0.017), and smaller total healing time of the lesion in patients in the intervention group compared to the control group (odds ratio, 1.98 [CI, 1.12 to 3.48]; p = 0.018).
According to Di Franco (2012), the use of food supplement based on antioxidants exerts a protective effect by decreasing skin toxicity in patients undergoing RT for breast cancer [10]. Studies relating the use of ONS with specific nutrients that helps in the prevention and/or treatment of radiodermatitis have not yet been described in the literature.
Considering the importance of an adequate intake of macro and micronutrients for the prevention and healing process, a study of the use of ONS with nutrients that acts in the healing process to help the prevention/treatment of radiodermatitis is relevant.
The aim of this case report was to evaluate the effect of ONS with nutrients that help in the healing process, in the prevention and/or treatment of radiodermatitis in patients undergoing RT for breast cancer.
Three patients were followed during adjuvant RT treatment for breast cancer at a Cancer Center in the city of São Paulo. All patients underwent 25 sessions of RT in the 3D technique. Anamnesis, age, cancer diagnosis, comorbidities and previous treatments were recorded. The patients were named in [1,2,3].
Patient 1 was instructed to start using the specific ONS for healing (Novasource Proline - Nestlé®) twice a day at the beginning of RT and maintain it until the end of the treatment; patient 2 started the specific ONS for healing after detecting grade I radiodermatitis and continued to use it until the end of the treatment; and patient 3 did not use any type of nutritional supplementation, being the control.The assessment of nutritional status was performed using bioelectrical analysis (BIA), applied at three times during the study on the day of RT planning, in the 16th session and in the 25th session of RT
For the BIA assessment, the patients were instructed to have the last meal two hours before the exam, not having metal plates and/ or pacemaker, not having performed vigorous physical activity in the last 12 hours and not having consumed large amounts of caffeine. The data generated by the BIA and used in the study were percentage of lean body mass (%LM), percentage of fat mass (%FM) and phase angle (PA)
The BMI (Body Mass Index) calculated by the formula BMI = Weight / (height) 2 was also evaluated. Weight was measured on a Filizola® scale, with the participant standing, barefoot and heels together. Height was measured with a Filizola® stadiometer with the participant standing, barefoot, heels together, head erect with eyes fixed in front of the body. The reference value used to classify the BMI for the participants was from the WHO (1997) [11]
The radiodermatitis assessment was performed weekly by the RT nursing team, and its classification was performed using the scale proposed by the RTOG (The Radiation Therapy Oncology Group, 1985) [12]. The patients were instructed to use a neutral moisturizer, without alcohol and without oil, in the treated region, three times a day from the tenth RT session and assess the nursing staff if they presented toxicity, such as burning or itching, at any time of the treatment. On the last day of treatment, they consulted with the RT nursing team, according to the department’s routine, for skin evaluation and guidance as needed.
Case report approved by the Research Ethics Committee of Fundação Antonio Prudente - A.C. Camargo Cancer Center under code RC 92/20. All patients signed an informed consent form for the publication of their clinical cases.
The patients 1 and 2 who used the ONS had good acceptance and tolerance which was considered an ingestion of >70% as the ONS prescribed during the entire period. The results in table 1 showed that patients 1 and 2 had a beneficial result for the onset of radiodermatitis and evolution of its degree when compared to patient 3 (control).
| Presence and grade of radiodermatitis | |||
|---|---|---|---|
| Patient | RT planning | 16th session of RT |
25th session of RT |
| 1 | Not present | Not present | Present/Grade I |
| 2 | Not present | Present/Grade I | Present/Grade I |
| 3 | Not present | Present/Grade I | Present/Grade II |
RT: radiotherapy.
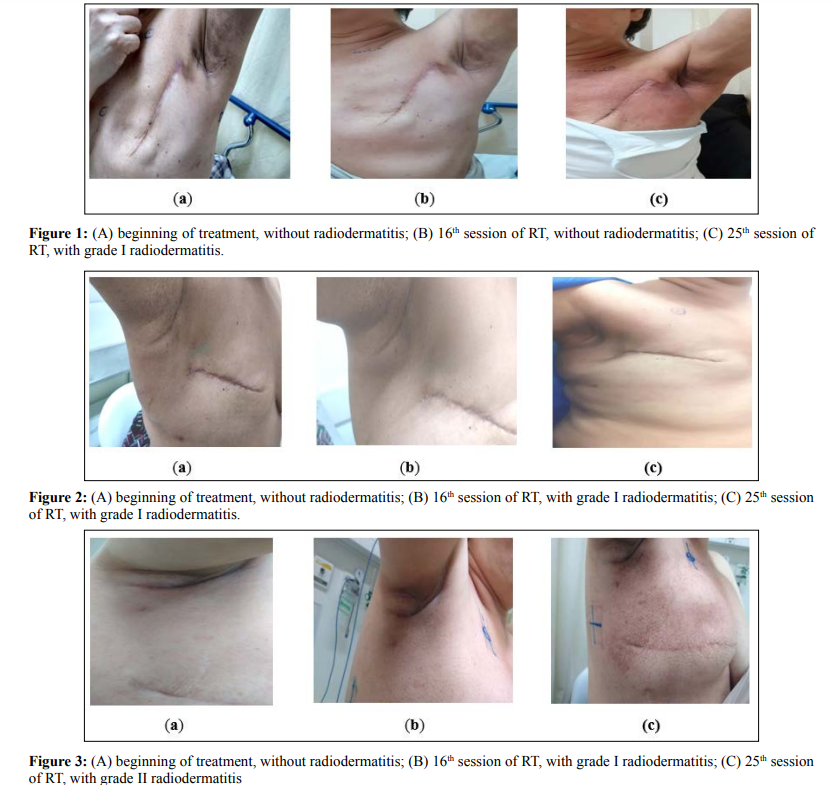
Patient 1, who underwent ONS prophylaxis, had radiodermatitis only at the 25th session of RT with grade I toxicity (Figure 1). Patient 2 presented radiodermatitis in the 16th session of the treatment, where the consumption of the ONS started. The toxicity assessed by the nursing staff was grade I and the lesion did not progress until the end of treatment (Figure 2). Patient 3 (control), presented grade I of radiodermatitis in the 10th session, evolving to grade II in the 23rd session (Figure 3).
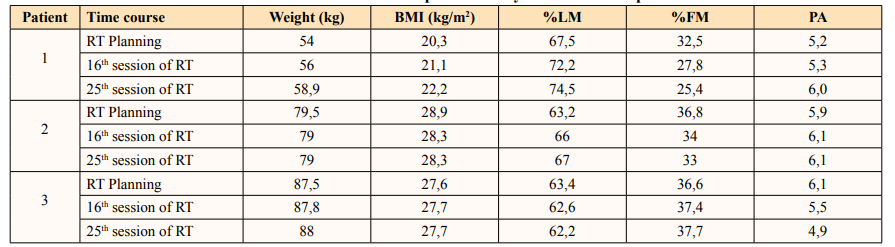
Regarding nutritional status (Table 2), patient 1 showed weight gain with improved body com-position, while patient 2 maintained both weight and the distribution of fat mass and lean mass. The control patient (3) presented weight maintenance but a slight change in lean and fat mass, influencing body composition. The phase angle obtained by BIA also showed important differences between the 3 cases studied, showing improvement when using specialized ONS for prophylaxis and maintenance when using specialized ONS for treatment.
BMI: Body Mass Index; LM: lean mass; FM: fat mass; PA: phase angle
Radiotherapy (RT) induced skin toxicity is still a major clinical problem that affects patients with breast cancer undergoing RT. Thus, prevention and treatment that can protect organs from the side effects induced by RT are increasingly relevant [13].
The patients followed in the present study showed relevant differences regarding the onset and degree of toxicity. Patient 1, who started supplementation from the first day of treatment, had a delay in the onset of radiodermatitis, which started only in the 25th session of RT and in grade I, not progressing to higher degrees. Patient 2 started supplementation after the development of grade I radiodermatitis, which occurred in the 16th session and did not show any worsening of toxicity, maintaining grade I until the end of treatment. On the other hand, patient 3, who did not receive supplementation, presented grade I radiodermatitis in the 10th treatment session, evolving to grade II toxicity. These results corroborate what was described in a study carried out by Di Franco et al (2012), which showed a benefit in the use of a food supplement containing antioxidants, important compounds that help in healing for patients undergoing breast RT treatment [12].
The result of the phase angle obtained during follow-up shows that the value has been maintained or increased in patients who used specific oral nutritional supplementation. On the other hand, the patient who did not use supplementation had this value significantly decreased during treatment. According to Norman (2014), the phase angle (PA), obtained through bioelectrical impedance (BIA), has been interpreted as a superior prognostic index of cell membrane integrity, and that a low PA is associated with worse prognosis in patients with cancer or serious. The author also describes that cancer patients whose PA is below the fifth reference percentile have significantly higher mortality at 6 months, even if adjusted for other potential risk factors, such as disease severity and weight loss [14].
Although there are still no studies showing the benefits of oral nutritional supplementation, whether specific or not, in reducing the incidence or severity of radiodermatitis, it is speculated that, since specific nutrients, such as arginine and antioxidants, help in the treatment of lesions of the skin, could contribute to the control of radiodermatitis.It is known that oral nutritional supplements are alternatives for increasing food intake and reducing weight loss in cancer pa-tients. However, the formulas are not always aimed at regaining weight, and they can be used to increase the supply of specific nutrients that may present deficits, as in the treatment of pressure injuries.
As previously mentioned, arginine, glutamine and antioxidants act in wound healing and can be an alternative for the prevention and/or treatment of radiodermatitis.
Large studies are necessary to demonstrate the benefits of using nutritional formulas with specific nutrients in reducing the incidence and/or severity of radiodermatitis.
In this case report we showed that the use of oral nutritional supplements with specific nutrients helps in the healing process of radiodermatitis in patients undergoing RT in the breast region, in addition to maintaining/improving body composition. More studies are necessary to confirm this hypothesis.
Author Contributions: Conceptualization, T.M. and R.C.; Methodology, T.M. and R.C.; Validation, T.M. and R.C.; Formal analysis, T.M., S.D. and M.M.; Investigation, T.M., S.D. and M.M.; Resources, R.C.; data curation, T.M., S.D. and M.M.; writing-original draft preparation, T.M and S.D.; writing- review and editing, T.M., S.D., M.M. and R.C.; Supervision, T.M and R.C.; project administration, T.M and R.C.; funding acquisition, R.C. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Fundação Antonio Prudente - A.C. Camargo Cancer Center, protocol code RC 92/20 (05/04/2021).
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper” if applicable.
Acknowledgments: To Nestlé Health Science for providing the oral nutritional supplement.
Conflicts of Interest: I, Thais Miola, first author of the manuscript entitled Case Report - Effects of specialized supplementation in the healing process for the prevention / treatment of radiodermatitis, and all co-authors who present themselves here, declare that we have a conflict of interest in the design and decision to publish the results of this case report.
