Author(s): Isaias Perez Negrete
ABSTRACT
Meningiomas are among the most common primary benign tumors and most of them are primary intradural lesions located in the subdural space. According to Casas-Perera et al., they represent 36.4% of the primary intracranial tumors. Meningiomas may be incidental, small, and slow growing tumors or extensive and/or rapidly progressive growing masses. There is a subset of meningiomas that arise outside the intradural space, called Primary Extradural Meningiomas (PEM) which, as the name suggest, develop outside the intracranial compartment and in this myriad of locations, Primary Intraosseous Meningiomas (PIOMs) are considered an uncommon type of PEMs that constitute up to 2% of meningiomas overall.
It is important to highlight the fact that PIOMs, in a different fashion as intradural meningiomas manifest, present themselves with a variable radiological appearance and clinical behavior as classical intradural meningiomas do. The treatment usually consist of complete en-bloc resection, and as straightforward as may seem at first glance, this goal may be challenging particularly due to the lack of clear tumor margins on conventional imaging studies .
The lack of a clear and standardized definition of this family of tumors remain within the domain of confusion. This is even more patent in the pediatric population, where except for very few report cases and specialized literature most of them fail to mention even an incidence or frequency in pediatric patients.
We present a case study of a 15-year-old male patient that suffered of refractory to anti-epileptic drugs (AED) seizures in order to attempt a resection.
A 15-year-old male patient came to office consultation due to a history of mild intellectual disability, seizures and an obvious left frontoparietal bone tumour covered with healthy scalp in order to evaluate surgical resection. The CT revealed a pure intraosseous tumour of up to 35 mm thick, and a 10*9 cm long (Figure 1), slightly left sided in the frontoparietal junction that crossed the midline over the Superior Sagittal Sinus (SSS) and caused significant subjacent brain oedema [1-8].
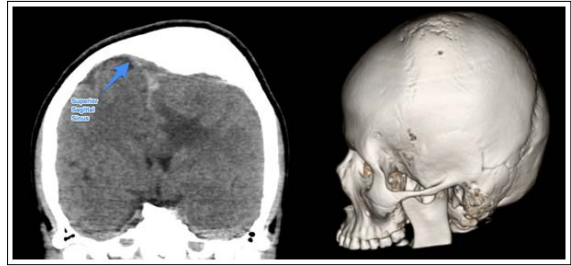
Figure 1: Left: coronal CT scan that shows the relationship between the vault, the Superior Sagital Sinus and the brain parenchyma underneath. Right: A 3D model reconstruction depicting the affected area and the obvious deformed area
We performed a centered Souttar skin incision in order to expose the exophytic visible extension of the affected bones, performed multiple drills surrounding the affected bone, about two centimetres away the obvious margins of the tumour and filled them with bone wax. After cautiously dissecting the epidural space, being specially gentle with the midline, we used high speed craniotome to cut the bone and removed all the tumour en bloc (Figure. 2) then we proceeded to control the profuse haemorrhage with haemostatic sponge, generous saline solution, compression and suction. Subsequently we placed a titanium mesh (Figure. 3) and finished the procedure with a blood loss of 400 cc and the patient was discharged the day after.
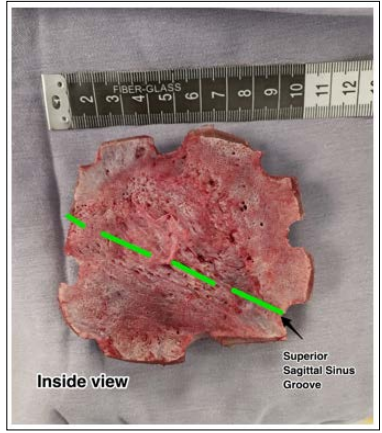
Figure 2: En bloc ressected cranial vault with a highlited path of the SSS. Note the obvious deformation caused by the tumour within
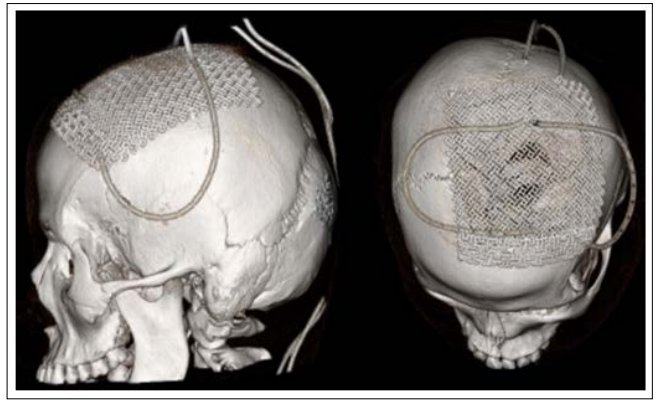
Figure 3: 3D CT showing the titanium mesh placed over the large bone defect
The patient came 2 months after surgery to office consultation with a good cosmetic outcome, his speech was slightly more fluent and just suffered one seizure a week after discharge (Fig 4). The pathology department reported a transitional intraosseous meningioma (WHO Grade I) (Figure. 5) free of neoplastic malignant cells, cellular atypia or mitosis. The patient did not undergo any adjuvant therapy since the pathology department report suggested that the borders of the pathological sample showed no tumoral activity. 14 months later, the patient did not exhibit any sign of tumoral reactivation nor rejection of the titanium mesh, still, he suffered up to 4 seizures in a year which the family considered as a great improvement over his basal state of seizures refractivity. A month later the patient was definitively discharged.
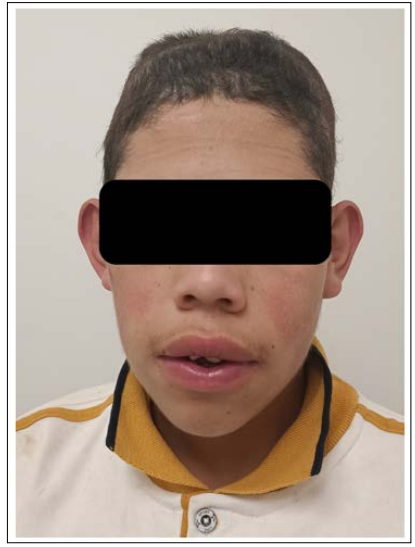
Figure 4: The patient in his office consultation two months after discharge. During this time, the patient ameliorated his seizure activity, improved his speech skills and showed a good cosmetic outcome
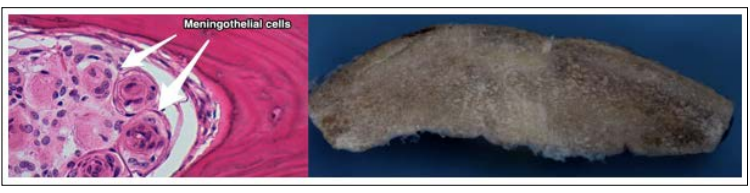
Figure 5: Left: a 40x HE stain that reveals a typical meningothelial nest common in these meningiomas. Right: Macroscopical view of the resected bone. Note the intradiploic proliferation and augmented thickness of the affected bone
PIOMs are rare, even more so in paediatric population as stated in the introduction. Most of the literature mention that intradiploic meningiomas tend to be benign in nature, however Richardson et al., reported a malignant PIOM in a 16-year-olfd girl in the United States for the first time in 2018 [1]. It is important to bear in mind differential diagnoses such as Osteoma, fibrous dysplasia, osteoblastic skull metastasis, Paget disease, among others [6]. We would like to remark that PIOMs are histologically and immunophenotypically indistinguishable from their intracranial counterparts and most common histologic subtypes include meningotheliomatous (62%), transitional (25%), fibroblastic (8%),
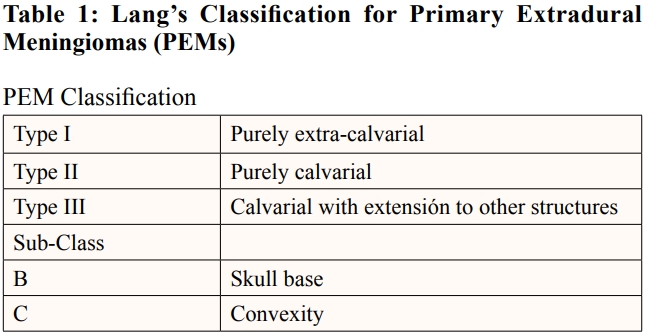
PIOMs are not new at all, since they have been described for the first time in 1904 [11]. A definition for PIOMs could be meningiomas with the largest component contained within the calvarium with no or only minimal dural involvement [5]. Despite not being expressively designed for PEM in pediatric patients, there is a classification for general PIOMs designed for Lang et als in 2000: A Type I PEM does not involve the skull and is termed purely extra-calvarial [12]. A Type II PEM is confined to the skull and is termed purely calvarial. Type III includes PEMs that involve the skull, but also extend to other structures. PIOMs that involve the skull are sub-classified as involving the convexity
Whenever we refer to these tumors as “primary” is not a matter of choice, since this term differentiates extension or metastasis from an intradural meningioma that could be otherwise called “secondary extended intraosseous meningioma”. This also accounts for ambiguity of extracranial that would exclude spinal and include ventricular [13].
PIOMs typically present as a slowly expanding painless scalp mass, with a possible relationship to a cranial sutures [5]. Common locations include the periorbital and frontoparietal regions [14]. The next most common presenting symptom is a neurodeficit (30%) [5]. Neurological symptoms are largely based on their location. This patient came to primary care unit because of a long history of refractory to AED seizures, mild mental retardation and an obvious cosmetic deformity that grew rapidly over the past 3 years. Despite the apparent oedema on CT scan the patient showed no sign of motor deficit.
As a matter of fact, gross total resection whenever possible is a curative treatment alone [1, 12, 14]. Although adjuvant therapy is frequently used, especially if there is an incomplete resection, or if the tumor was atypical or malignant [15]. It is advisable to attempt a reconstruction of the resected bone with the best option suitable for the patient age, since titanium mesh should not be used for patients under 12-year-old it is advisable to use autologous bone graft harvested from rib or iliac bone, this last option suitable only in children over 6-years-old since the potential adverse effect in growth impairment [16, 17].
It is difficult to stablish in the near future a series of cases of pediatric PIOMs since they are very scarce and most of the authors (if not all) present just case reports. Nevertheless, many histological features, location, clinical presentation, treatment and prognosis are similar to the adult counterparts, thus the decision making in the operation room could be shared as well in both populations. Perhaps the most important difference during intervention could be the potential reconstruction of the bone deprived area, since as we discussed in earlier paragraphs, the vault reconstruction should be tailored for age dependent reasons rather than prosthetic availability.
I would like to thank Dr Luis Humberto Cruz Contreras for his contribution as the Pathologist responsible of this case.
I hereby declare that the present paper does not have any commercial, economic, academic nor political conflict of interest.
1.Richardson B, Lammle M, Laurini J, Martino A (2018) Malignant primary intraosseous meningioma in a pediatric patient: A case report and review. Interdiscip Neurosurg 14: 156-160.
2.Casas Parera I, Báez A, Banfi N, Blumenkrantz Y, Halfon MJ, et al. (2016) Meningiomas en neurooncología. Neurologia Argentina 8: 210-226.
3.Dires Fenta B, Israel Korga T, Dinka Bikila T, Kassahun Tadele A, Lijalem Yigezu B, et al. (2022) Primary Intraosseous Meningioma: Bifrontal Skull Mass. Pathol Lab Med Int 14: 25-31.
4.Guinto-Nishimura GY, Gómez-Amador JL, Kerik-Rotenberg N, Uribe-Pacheco R, Sangrador-Deitos MV, et al. (2021) 68Ga-DOTATOC-PET/CT–guided resection of a primary intraosseous meningioma: technical note. Neurosurg Focus 50: 1-7.
5.Arana E, Menor F, Lloret RM (1996) Intraosseous meningioma. J Neurosurg 85: 362-363.
6.Choudhary G, Udayasankar U, Saade C, Winegar B, Maroun G, et al. (2019) A systematic approach in the diagnosis of paediatric skull lesions: What radiologists need to know. Vol. 84, Polish Journal of Radiology. Medical Science International e92-111.
7.Mahmoud Reza Khalatbari, Hamid Borghei-Razavi, Yashar Moharamzad (2011) Intradiploic orbital roof meningioma with pneumosinus dilatans in a child: a case report and review of the literature. Pediatr Neurosurg 47: 66-71.
8.Madjid Samii, Mario Ammirati (1992) Surgery of skull base meningiomas. 1st ed. Heidelberg: Springer Berlin, Heidelberg 117-121.
9.Herrera Armendáriz G, Ramírez Sánchez D, Guadalupe R, Rosales P, Rangel CC (45) Primary intraosseous meningioma. Case report and literature review Meningeoma intraoseo primario. Revista Chilena de Neurocirugía 45.
10.Yamazaki T, Yamazaki T, Tsukada A, Uemura K, Satou H, et al. (2001) Intraosseous Meningioma of the Posterior Fossa- Case Report 41.
11.V SB, Roy K, Banga MS, Ghosh P (2016) Intradiploic meningioma- A rare osteolytic calvarial lesion: A case report and review of literature. Asian J Med Sci 7: 103-105.
12.Lang FF, Macdonald OK, Fuller GN, DeMonte F (2000) Primary extradural meningiomas: a report on nine cases and review of the literature from the era of computerized tomography scanning. J Neurosurg 93: 940-950.
13.Elder JB, Atkinson R, Zee CS, Chen TC (2007) Primary intraosseous meningioma. Neurosurg Focus 23: E13.
14.Crawford TS, Kleinschmidt-DeMasters BK, Lillehei KO (1995) Primary intraosseous meningioma. Case report. J Neurosurg 83: 912-915.
15.Partington MD, Scheithauer BW, Piepgras DG (1995) Carcinoembryonic antigen production associated with an osteolytic meningioma. Case report. J Neurosurg 82: 489-492.
16.Tokgoz N, Oner YA, Kaymaz M, Ucar M, Yilmaz G, et al. (2005) Primary Intraosseous Meningioma: CT and MRI Appearance. AJNR Am J Neuroradiol 26: 2053-2056.
17.Sahoo NK, Roy ID, Rangarajan H (2011) Cranioplasty in children with split rib graft. Med J Armed Forces India 67: 83-85.
View PDF