Author(s): Hassan Akouch*, Malek Michael Bouhairie, Sabrina Nasreddine, Racha Seblani, Maryam Bouhairie Kreidly and Ahmad Mroue
Introduction: Drug induced liver injury or DILI is any injury to the liver by a medication, herb, or dietary supplement. Ranking as the first cause of acute liver failure in the USA and Europe, spectrum of clinical presentation may range from asymptomatic elevated liver function test to ALF. Approximately 20 new cases of DILI per 100,000 persons occur each year worldwide. Classified as intrinsic (with the most common cause being acetaminophen), and idiosyncratic adverse drug reaction (including mostly those related to antibiotics, NSAID drugs, and isoniazid).
Isotretinoin is indicated to treat severe inflammatory acne that is refractory to antibiotics or topical agents; Although it has a high margin of safety, adverse effects include transaminasitis, like many retinoids, but unlike acitretin and etretinate, isotretinoin has not been clearly implicated in cases of clinically apparent acute liver failure.
We report a case of 31 year old lady on isotretinoin therapy for her acnea since 8 month with poor follow up, presenting with acute liver failure to our emergency department.
Case Presentation: 31 year old lady , NKDFA, on isotretinoin for her acne, started 8 month ago at a dose 40mg daily, is brought by her family for decrease level of consciousness and increasing jaundice since around 5 days associated with mild abdominal disconfort. Intubated for GCS of 3, laboratory tests showed prolonged INR and elevated total bilirubin, mainly direct, with elevated transaminase levels, all work up for other etiologies turned negative, and patient was diagnosed with isotretinoin inducing acute liver failure.
Discussion: Hepatotoxicity manifesting by liver test abnormalities, occur in up to 15% of patients on isotretinoin. These liver test abnormalities are usually asymptomatic and resolve spontaneously even without discontinuation of therapy in most cases. Severe liver injury due to isotretinoin is exceedingly rare: The acute liver failure was only been described with etretinate and acitretin and not with isotretinoin therapy.
Risk factors for DILI include older age, female sex, African American, pharmacological risk (including daily dosage, degree of lipophilicity and extent of hepatic metabolism), preexisting liver disease and Host Genetic Factors. An important association was found between the dose of oral medication and hepatotoxicity in the United States and Sweden, in addition to a positive association between higher drug lipophilicity and DILI in condition to be coupled with high dose ingestion. Our patient meets the criteria for sex and for the pharmacological characteristic of isotretinoin (which is a highly lipophilic drug and was ingested at 40mg daily).
DILI may cause cholestatic or hepatocellular liver injury or mixed on the basis of the R value, In addition, studies have showed that DILI in females is more often hepatocellular and may be associated with a more severe course, which can result in the need for liver transplant, or death and all that were compatible with our case.
As the disorder is rare, there are no specific biomarkers for diagnosis of idiosyncratic DILI, and diagnosis is made by exclusion.
Recent advances in the diagnosis of DILI include the recognition of the importance of the establishment of clinical networks to refine causality assessment estimated by RUCAM score and also the use of expert panels in the diagnosis of DILI [3]. The calculated RUCAM score for our case is equal to 8, indicating probable drug reaction.
Concerning acute liver failure, the most widely accepted definition from the American Association for the Study of Liver Diseases (AASLD) is ‘’evidence of coagulation abnormality, usually an international normalized ratio above 1.5, and any degree of mental alteration (encephalopathy) in a patient without preexisting liver disease and with an illness of less than 26 weeks’ duration’’. Based on all above, the presentation of our patient was typical for an acute liver failure induced by the drug isotretinoin.
The only curative treatment of drug induced acute liver failure is liver transplantation.
Conclusion: This is probably the first case reporting an acute liver failure induced by isotretinoin therapy. Strict monitoring of liver tests is highly recommended for patients receiving isotretinoin at regular intervals, with close observation and follow up, because, although rare, it may induce an acute liver failure with deleterious results. Future works must include a discovery of an early markers of DILI, for early detection and prevention in the high risk patients.
Acute Liver Failure, Ro-Accutane/Isotretinoin Therapy, Liver Transplant, Hepatitis, Encephalopathy, Drug Induced Liver Failure, Case Report
DILI, drug induced liver injury, ALF, Acute Liver Failure, NSAID, non steroidal anti-inflamatory, NKDFA, not known drug or food allergy, GSC, glascow coma scale, INR, international normalized ratio, APAP, N-acetyl-para-aminophenol, ALT, alanine aminotransferase, AST, aspartate transaminase, LFT’s, liver function tests, LOC, loss of consciousness, PMH, past medical history, PSH, past surgical history, HR, heart rate, BP, blood pressure, ICU, intensive care unit, IV, intravenous, NSS, normal saline, PNG, per nasogastric tube, IVD, intravenous drip, FFP, fresh frozen plasma, PPI, proton pump inhibitor, HAV, hepatitis A virus, HCV, hepatitis C virus, HIV, human immunodeficiency virus, HBs Ag, hepatitis B surface antigen, ANA, antinuclear antibody, MSSA, methicillin sensitive staphylococcus aureus, DTA, deep tracheal aspiration, IBD, inflammatory bowel disease, ULN, upper limit normal, NAC, N-acetyl-cystein.
Drug induced liver injury or DILI is any injury to the liver by a medication, herb, or dietary supplement [1]. Responsible for 3 to 5% of hospital admissions for jaundice, it represents nowadays a major health problem due to the increase exposure of humans to drugs and herbal medicines [2,3]. Between 1969 and 2002, it was the main reason of 15% of drugs withdrawn from the market [2]. Ranking as the first cause of acute liver failure in most Western countries, accounting for more than half of cases, the spectrum of clinical presentation may range from asymptomatic liver test elevations to ALF [3]. Approximately 20 new cases of DILI per 100,000 persons occur each year worldwide [1-3].
Classified as either direct, idiosyncratic and newly emerging indirect injury type. Direct hepatotoxicity injury is characterized by its predictability, the dose-dependence, the reproducibility in animal models and the short latency period usually within 1 to 5 days following ingestion of an overdose (intentionaly or accidentaly) of a drug that is intrinsically toxic to the liver by its direct chemical components or its metabolites. The most representative example of this type of liver damage is the acetaminophen (APAP) induced hepatotoxicity considered the most common cause of drug induced acute liver failure occurring in around 40% of patients in US [2,3]. To note that non-APAP drug injury represents 11% of all cases of DILI in the latest registry from the US ALF Study Group [4].
In contrast, idiosyncratic adverse drug reaction, one of the most challenging diagnoses faced by clinicians because of the great variety of drugs capable of damaging the liver and their different clinical aspect, are unpredictable, not dose dependent and difficult to prevent and to diagnose due to the current lack of diagnostic biomarkers. The latency period generally ranges from 5 to 90 days. Overdosing does not induce liver injury in the majority of patients although there seems to be a dose threshold. According to the R ratio, idiosyncratic hepatotoxicity is categorized as hepatocellular (R value more than 5), cholestatic (R value less than 2) or mixed (R value between 2 to 5); R value calculated by dividing the alanine aminotransferase level by the alkaline phosphatase level in correspondence to the upper limit normal range of each [2]. The hepatocellular type is the most common manifestation of idiosyncratic liver injury with a high mortality especially when coupled with jaundice forming the Hy’s law. Common causes of drug-induced idiosyncratic acute hepatocellular injury are isoniazid, amoxicillin/clavulanate, nitrofurantoin, and NSAID [1-5].
Concerning the Indirect hepatotoxicity type, liver injury is due to the action of the drug where it can induce an immune mediated hepatitis in a previously healthy liver or an exacerbation of a preexisting liver disease [3].
Acne, one of the commonest reason for dermatological consultations, is a chronic inflammatory disorder of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world. The treatment of acne depends on its severity: in the non-inflammatory or mild inflammatory disease, use of topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics is applied. In cases of severe inflammatory acne, such as nodulocystic or conglobata acne, or cases refractory to conventional treatment, systemic isotretinoin is recommended [6-8].
Approved for use in acne in the United States, in 1982, but only under strict requirements for monitoring and birth control, isotretinoin, also known as 13-cis-retinoic acid, is an aromatic retinoid and vitamin A derivative, highly lipophilic drug, not stored in the liver and is not associated with many of the toxic effects of high dose vitamin A therapy. It acts by activating the complex retinoic acid-retinoid X receptors responsible for regulation of gene expression important in normalizing cell growth and differentiation leading to keratinization of the sebaceous follicles. Dosages range from 0.5 to 2.0 mg/kg per day given in two divided doses for 15 to 20 weeks with higher doses have been used in treatment of head and neck cancers [7-9].
Although a high margin of safety is present, this drug has several adverse effects with the most significant consisting of teratogenicity and muco-cutaneous adverse effects such as cracked lips, dryness of the skin, nose and eyes. It was also linked to cause depression, psychosis, suicide, headache and vomiting. In addition, it may lead to alteration in the lipids, including increased total cholesterol and low-density lipoprotein (LDL) cholesterol levels, increased triglyceride and reduced levels of high density lipoprotein (HDL) cholesterol. Also Isotretinoin, like many retinoids, can lead to hepatitis manifesting in the form of transaminitis, but, unlike acitretin and etretinate, isotretinoin has not been clearly implicated in cases of clinically apparent acute liver injury with jaundice. Due to the clinical significance of the possible adverse reactions caused by isotretinoin, recommendation is to repeat ALT, AST and triglyceride measurements after 30 days from starting therapy and then every three months thereafter with close follow up [6-9]. The majority of dermatologists, around 89%, would stop isotretinoin if LFTs were four times the normal range, and around 72% would end the course of the treatment if triglyceride levels were four times the normal range [10].
We report a case of 31 year old lady on isotretinoin therapy for her acne since 8 month with poor follow up, presenting with acute liver failure to our emergency department.
A 31 year old lady, NKDFA, is brought by her family to our emergency department for decrease level of consciousness. Family mentioned progressive decrease in LOC in those 2 days, and noted a jaundice since around 5 days associated with mild abdominal pain. Her only home medication is isotretinoin for her acne, started 8 month ago at a dose 40mg daily with no laboratory follow up since last 7 month.
PMH: acne
PSH: none
Family history: negative for liver disease
Habits history: smoker, non-alcoholic
On physical examination, she was afebrile, hemodynamically stable, with HR 89 and BP 120/65mmHg. Patient is somnolent/ coma, no response to pain stimuli, GCS of 3, bilateral mydriasis with icteric conjunctiva and jaundice covering all her body; the abdominal exam was soft with positive bowel sound; No hepatosplenomegaly, no other significant sign. Elective intubation was done, urgent scan abdomen pelvis without contrast done and findings were within normal range; Admitted to ICU, a foley catheter was inserted for accurate in/out, with full laboratory test done including liver function test that showed hepatocellular injury with elevated total bilirubin (16,6mg/dl) and prolonged INR (6,94) with activity of 12%. IV hydration with NSS 3l/24h started, with 400mg q6h of N-acetylcystein PNG and 1 vial of albumin 20% IVD per 12 hours; 10mg of Vitamin K IVD given once, with FFPs 2 unit Q6h started. In addition, lactulose PNG and ciprofloxacin started with PPI prophylaxis. Serology for HAV, HCV, HIV and HBS antigen and ANA level, all turned negative. Amonia level was elevated (98 umol/l). During her stay, patient started fever at day 3 of hospitalization, with new pneumonia at her left lung’s lower lobe on her chest radiography; pan culture taken that showed bacteremia with MSSA, DTA culture positive for staphylococcus aureus and candida albicans, and urin culture positive for candida albicans. At day 4, patient started to do recurrent clonic seizure suppressed by antiepileptic, urgent CT scan of brain done that did not show signs of increasing intracranial pressure or any bleed or mass effect. To note that during her stay no improvement in her neurologic exam was noted, despite giving 4 bowel movement per day. At day 7, the patient was transferred to ICU care at liver transplant center: clinical deterioration in her condition occurred with septic shock requiring vasopressors despite escalation of her antibiotics. Due to her clinical unstability, the plan for liver transplant from her sister was aborted.
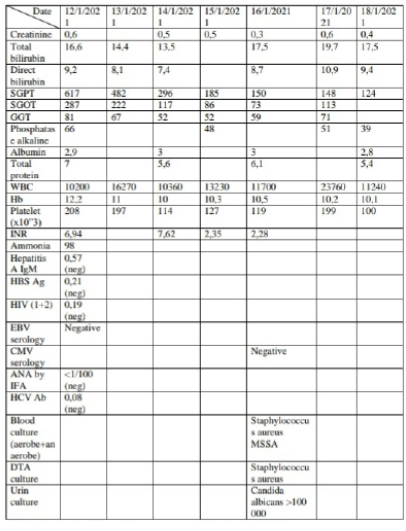
An acute liver failure arose during isotretinoin therapy in a young lady with no past medical history except for her acne.
The hepatic toxicity of vitamin A is well known, even a chronic use of a low therapeutic dose may lead to severe life-threatening liver damage [11]. Unlike vitamin A, isotretinoin, an aromatic retinoid which is effective in treating refractory nodular acne, is not stored in the liver explaining its less side effects compared to high dose vitamin A therapy [9]. Mainly its side effect include teratogenicity, IBD, depression and suicide; in addition it may cause worsening hyperlipidemia, dry skin, conjunctivitis and hair loss [7]. Hepatotoxicity manifesting by liver test abnormalities occur in up to 15% of patients on isotretinoin, although marked elevations of transaminase level requiring drug discontinuation are rare (<1%). These liver test abnormalities are usually asymptomatic and resolve spontaneously even without discontinuation of therapy. Liver injury due to isotretinoin is exceedingly rare: The acute liver injury was only been described with etretinate and acitretin and not with isotretinoin therapy. Probably due to strict requirements for close monitoring, once prescribed, and early discontinuation once transaminase level increase to more than 3 times ULN, the majority of reported cases of liver injury attributed to isotretinoin have been anicteric with no or minimal symptoms [9].
Our patient did only once liver function test, 1 month after starting therapy, that were normal as mentioned by her family, then she continued to take daily 40mg of isotretinoin with no follow up for 7 month until presentation. Drug induced liver injury (DILI) is the leading cause of ALF (more than 50% of overall cases) with the leading cause being acetaminophen/APAP (mainly from unintentional overdose); According to the latest registry from the US ALF Study Group (US ALF SG), non-APAP drug injury represents 11% of all cases with isoniazid presentingthe most common agent in a US registry, in addition to amoxicillin/clavulanate and nonsteroidal anti-inflammatory drugs [1,3,4].
Our patient did only once liver function test, 1 month after starting therapy, that were normal as mentioned by her family, then she continued to take daily 40mg of isotretinoin with no follow up for 7 month until presentation. Drug induced liver injury (DILI) is the leading cause of ALF (more than 50% of overall cases) with the leading cause being acetaminophen/APAP (mainly from unintentional overdose); According to the latest registry from the US ALF Study Group (US ALF SG), non-APAP drug injury represents 11% of all cases with isoniazid presentingthe most common agent in a US registry, in addition to amoxicillin/clavulanate and nonsteroidal anti-inflammatory drugs [1,3,4].
DILI may cause cholestatic or hepatocellular liver injury or mixed on the basis of liver biochemical parameters. These 2 subtypes are categorized according to a formulas defined by the Council for International Organizations of Medical Sciences and modified by the Food and Drug Administration (FDA): the R ratio, which is the ratio of the alanine aminotransferase (ALT) to the alkaline phosphatase normalized to their respective upper limits of normal (ULN).The R ratio for hepatocellular DILI is more than 5, for cholestatic DILI is less than 2, and between 2 and 5 for mixed type [1,3]. The R ratio calculated in our patient is (617/42) divided by (66/135) = 30 indicating an hepatocellular injury. In addition, studies have showed that DILI in females is more often hepatocellular and may be associated with a more severe course, which can result in the need for liver transplant, or death and all that were compatible with our case.
As the disorder is rare, there are no specific biomarkers for diagnosis of idiosyncratic DILI, and clinical presentation is often non specific mimicking many other liver disorders, making the diagnosis very difficult to be established. It depends usually on clinical suspicion with the exclusion of the common causes of liver disorder: such as viral hepatitis (serology for HAV, HCV, HIV and the HBS antigen all turned negative excluding a viral hepatitis in our patient), alcohol (absence of history of alcohol use in our patient exclude this diagnosis), autoimmune liver disease (ANA level turned negative, in our patient, excluding the diagnosis of autoimmune hepatitis inducing liver injury), biliary obstruction (A CT scan abdomen pelvis was performed in our patient and had showed no evidence of biliary dilation in addition to the absence of cholestatic pattern calculated by R ratio that showed an hepatocellular injury), ischemia (the ratio of ALT over LDH in our patient is equal to 3,5 superior to 1,5 and so eliminating an ischemic cause; in addition there is no history or of likelihood episode of shock and patient not known to have a history of heart failure, all of that exclude this diagnosis) and sepsis (at presentation , our patient was hemodynamically stable in terms of BP and urine output excluding this diagnosis, later on she developed VAP complicated by bacteremia which led to the need of vasopressors). In addition, hypersensitivity inducing liver injury should be ruled out; Our patient was negative for eosinophilia, and did not complain of pruritus or any other sign of allergic reaction while on isotretinoin treatment making this diagnosis unlikely.
Recent advances in the diagnosis of DILI include the recognition
of the importance of the establishment of clinical networks to
refine causality assessment and also the use of expert panels in
the diagnosis of DILI. The standard causality assessment tool
for defining DILI, without liver histology, is a scoring system
to predict the probability of DILI known as the Roussel--Uclaf
causality assessment method (RUCAM). RUCAM is a superior
causality tool compared to the simpler clinical diagnostic scale
but inferior compared to expert panels to identify DILI which was
found to be more reliable than RUCAM, based on establishment
of consensus for multiples criteria about definition of DILI, the
type of liver injury, causality assessment, severity and chronicity
[5].
The calculated RUCAM score for our case is equal to 8,
indicating probable drug reaction calculation based on several
criteria: hepatocellular type on liver injury, second exposure, 8
month being the time from drug intake until reaction onset, with no
withdrawal of the drug during these 8 month, absence of alcohol
risk factor, age group less than 55 years old, course of the reaction
showed more than 50% improvement in the transaminase level
in less than 8 days interval, no other concomitant drug ingestion
as mentioned by her family, exclusion of non drug-related cause
as mentioned above, hepatitis labeled as a side effect in the
isotretinoin, and lastly the drug was removed upon presentation
with no readministration. Based on those criteria and according
to the calculator of RUCAM, the score turned [7,12].
Concerning acute liver failure, the most widely accepted definition from the American Association for the Study of Liver Diseases (AASLD) is ‘’evidence of coagulation abnormality, usually an international normalized ratio above 1.5, and any degree of mental alteration (encephalopathy) in a patient without preexisting liver disease and with an illness of less than 26 weeks’ duration’’. Our patient, with no preexisting liver disease, presented to our emergency department, after several days of jaundice , with a near coma status (encephalopathy grade 4) and prolonged INR at exam 6,94 [13].
Based on all above, the presentation of our patient was typical for an acute liver failure induced by the drug isotretinoin. There are no specific therapies available for DILI. The treatment of drug induced ALF, as for other causes of ALF, rely on supportive care and requires monitoring of the patient and laboratory work in an intensive care unit at a liver transplant center: our patient, upon presentation, was admitted to the ICU, and since her first day in the hospital, she was planned to be transfer to a liver transplant center, and the transfer occurred at day 7 for issue in admission and availability of places [1,3,9].
In addition, discontinuation of the offending agent is the first step (done with our patient since the day 1). In general, most DILI follows a benign course with liver function tests resolving on drug withdrawal, and in some rare cases it may lead to ALF as in our case [1, 3, 9].
If respiratory failure or grade 3 or 4 encephalopathy is present (as in our patient), airway protection and mechanical ventilation should be done with monitoring of intracranial pressure, and frequent neurologic examinations. If ALF present, patients should be worked up promptly for a potentially life-saving liver transplant. Like other causes of ALF, liver transplantation decreases the risk of death in patients who do not survive without advanced interventions [1, 3, 9]. In our patient, family was informed since the day 1 about the poor prognosis of the patient and for the urgent need for liver transplant, so genetic testing were done on her sister that found to be compatible for donation of a liver segment to the patient, but view the dramatic deterioration in the clinical status of the patient and the septic shock that had developed due to ventilator associated pneumonia caused by Staph aureus methicillin sensitive and complicated by bacteremia, the risk of surgical intervention for liver transplant was high and benefit versus risk were outweighted, so the transplantation did not occur as it is contraindicated in patient with concomitant active infection.
Corticosteroid use has not been proven to have benefit in ALF unless if caused by autoimmune hepatitis or linked to hypersensitivity form of liver injury, but there is no clear data for that practice for the later [1, 3, 9]. Accordingly, our patient did not receive steroid. Ursodeoxycholic acid therapy has been used in patients with cholestasis type DILI, but data supporting this agent remain limited [1, 3, 9]. View the hepatocellular pattern in our patient, ursodeoxycholic acid was not been administered.
The use of NAC, mainstay treatment for patients presenting early in their course of acetaminophen toxicity, has been recommended for possible use in non-APAP drug induced ALF in adults, improving mortality and the transplant free survival [1, 3, 9]. Our patient,
since day 1, was given N acetyl cysteine via NG tube.
Once acute liver failure induced by drug occur, the prognosis is very poor without a liver transplantation therapy with a survival rate of only 26%. Studies showed that the combination of hepatocellular type liver injury and jaundice, in the absence of any biliary disease, has been linked with a high mortality rate between 11.7% and 15%. This was known as ‘Hy’srule’ [1, 3, 9]. Our patient met this criteria.This is probably the first case reporting an acute liver failure induced by isotretinoin therapy. Non-APAP DILI is implicated in more than 1 in 10 cases of ALF in the United States. Fortunately, the risk of ALF from these drugs is low relative to their overall use, but once present, it carries a high mortality rate without liver transplantation necessitating an emergent multidisciplinary approach. So, strict monitoring of liver tests is highly recommended for patients receiving isotretinoin at regular intervals, with close observation and follow up, because, although rare, it may induce an acute liver failure with deleterious results. Future works must include a discovery of an early markers, probably better than the serum transaminase level, like the one in nowadays studies based on mRNA sequences, for early detection and prevention in the high risk patients [14].
We would like to thank the doctors and staff at our institution for their continuous support and guidance.
