Author(s): Luiz Eduardo Imbelloni* and Marildo A Gouveia
Background and Objectives: Severe complications such as Cauda Equina Syndrome (CES) lead to temporary or permanent disability have been ascribed to central neuraxial blocks. Some case reports, and very few retrospective studies, have focused their attention on the fact that central nerve blocks can cause, albeit rarely, permanent damage to the spinal cord or nerve roots, or both. This study is the experience of 48 years with spinal anesthesia and several publications and no definite neurological complications. This study is not a review of the subject. The primary objective of this study was to analyze the characteristics in relation to needles, puncture position, types of anesthetics, and baricity of local anesthetics.
Methods: This study on CES mainly on neuroaxial anesthesia complications was performed after searching using the key search words stated, using the Title, Abstract and Subject in six search sites (Pubmed, Science, Scielo, Lilacs, Medline, and Google Academic).
Results: The 12 phrases used for the survey found different responses depending on the site used. Several articles were studied, mainly correlated with neuraxial anesthesia.
Conclusions: The understanding of triggering factors of spinal anesthesia-induced cauda equina syndrome may prevent injuries and help early diagnosis and treatment, therefore changing patients prognosis. CES occurred with all anesthetics used in neuraxial blockade. The neurotoxicity of intrathecally administered of local anesthetic is increased by the addition of epinephrine, and is not influenced by the addition of glucose, puncture position, and typ of needles. CES was not found with hypobaric anesthetic.
• More common with orthopedic and neurological disorders, due to compression of the cauda equina nerve roots.
• The use of several forms of spinal anesthesia with hyperbaric, isobaric, hypobaric, continuous spinal anesthesia, thoracic spinal anesthesia, combined spinal-epidural anesthesia, unilateral spinal anesthesia, posterior spinal anesthesia, should be considered and the benefits of these techniquesfor different types of patients and surgeries must be known.
Cauda equina syndrome (CES) is rare, but it can have devastating consequences due to compression of the cauda equina nerve roots. This most commonly occurs due to a prolapsed intervertebral disc, therefore orthopedic and neurological problems [1,2].
There are several causes of CES, but the causes after neuraxial blockade (epidural, spinal or combined epidural-spinal anesthesia) will be addressed. Several local anesthetics injected into the neuraxial blockade (spinal or epidural) can cause CES such as 2-chloroprocaine (2% and 3%), 5% hyperbaric lidocaine, 0.5% hyperbaric bupivacaine, 0.5% hyperbaric tetracaine mixed with epinephrine, 0.5% hyperbaric bupivacaine and epidural anesthesia with ropivacaine, 2% lidocaine with epinephrine, 0.5% levobupivacaine, 0.75% hyperbaric bupivacaine with 15 μg of fentanyl, and 0.5% isobaric bupivacaine [3-11].
CES has been reported with all local anesthetics used in a single neuraxial injection as dibucaine, piperocaine, procaine, mepivacaine, tetracaine, lidocaine, bupivacaine, ropivacaine and lebobupivacaine, having been injected as a hyperbaric and isobaric solution. No CES with hypobaric solution of local anesthetics was found in the various search systems used. Likewise, punctures in the sitting or lateral position, with pencil point and cutting point needles, have been reported. In addition, there are reports of the use of a combined spinal-epidural block, and even with the association of ropivacaine, in addition to the use of an epidural with 2% lidocaine.
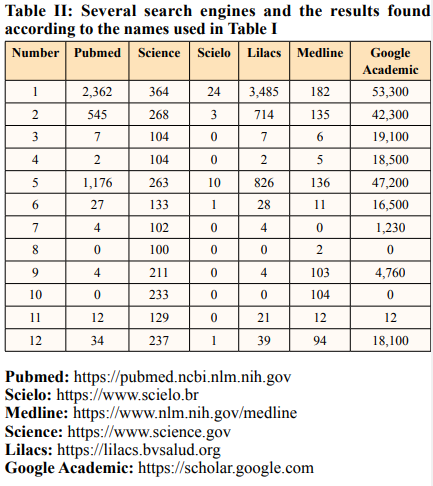
This study on CES mainly on neuroaxial anesthesia complications was performed after searching using the key search words stated (Table I), using the Title, Abstract and Subject in six search sites (Table II).
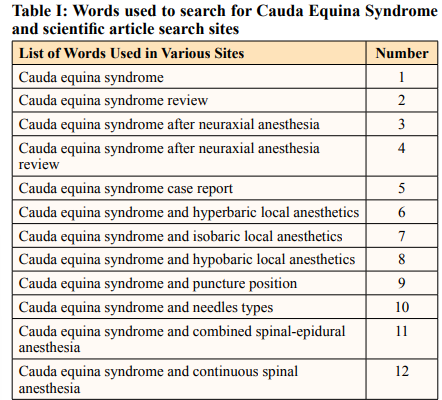
After the advent of the internet, it is common that the firstsource of search is Google, which is not at all common. However, it is not a search engine specialized in scientific articles, so Google Scholar (Academic) was created, which can bring more reliable sources. To facilitate the process of scientific research at universities, the US government created Science.gov, which integrates more than 60 databases from various agencies and bodies, having a huge collection that gives access to websites from more than 12 federal agencies. The Scientific Electronic Library Online (SciELO) platform was also used, which allows research and access to full texts of scientific journals from Central and South America. The LILACS website is a specialized database in the health area, with scientific and technical literature from 26 countries in Latin America and the Caribbean. MEDLINE is the premier bibliographic database that contains references to journal articles in life sciences, with a concentration on biomedicine. In this research on CES, the search through CAPES was not used, which is a virtual library with the best of Brazilian scientific production online.
The results of this CES survey across the six medical sites, with different results are all 12 phrases created for search [Table II]
CES is characterized by bladder and bowel dysfunction, loss of sensitivity in the perineal area and variable degrees of muscle weakness in the lower limbs, symptoms that begin immediately after the reversal of the subarachnoid block, and may be permanent or present partial, slow and progressive regression. gradual over periods ranging from weeks to months. Among the factors potentially capable of triggering it are direct or indirect trauma to nerve roots, ischemia, infection and neurological reactions, and toxicity of local anesthetics and other substances [12].
In 1997, in France, a survey was carried out using a questionnaire sent to 4,927 anesthesiologists with a response from 736 participants, and adverse events were reported in a cohort of 103,730 regional blocks, with permanent nerve damage occurredin 34 cases, and five cases were found (1.2:10,000) from CES [13]. It is important to emphasize that this study is a survey using a questionnaire, being cited by most authors who write about complications in the neuraxial, but none states that it is a survey
The use of intrathecal epinephrine has been implicated in a case report of cauda equina syndrome in a patient with vasculopathy [14]. Inrats the neurotoxicity of intrathecally administered lidocaine is increased by the addition of epinephrine [15]. Routine use of epinephrine in spinal anesthesia for patients with multiorgan vascular disease should be considered carefully because of the possibility of vascular insufficiency of the spinal cord which would be exaggerated by the vasoconstrictive effect of epinephrine. Since the appearance of 0.5% isobaric bupivacaine suggested in Editorial, and the decrease in the use of 5% hyperbaric lidocaine, the use of epinephrine has been discontinued by my group in Brazil [16]. The first scientific article was carried out with 0.5% isobaric bupivacaine in 1991 [17].
Reports of CES associated with the use of spinal catheters for continuous spinal anesthesia must always be questioned, as in the four cases described, three were performed with a 28G microcatheter and the use of high doses of 5% lidocaine with glucose, and one was performed with an epidural catheter ( 20G) and high doses of 0.5% hyperbaric tetracaine [18]. The physician and physicist Paracelsus in the Middle Ages already said that the difference between remedy and poison is the dose.
CES has been recognized as a rare, devastating complication of spinal anesthesia shown in 1937, in 14 patients undergoing anesthesia with heavy durocaine (procaine) [19]. In the introduction to this article, it was shown that all local anesthetics used on the neuroaxis caused CES, procaine, chloroprocaine, tetracaine, lidocaine, bupivacaine, ropivacaine, and levobupivacaine. However, the enantiomeric mixture of bupivacaine (R75:S25), developed and used in Brazil, there is no report of CES, both in spinal anesthesia and epidural anesthesia [20]. The use of levorotatory anesthetics both in animals and in humans proved to be less toxic than the racemic mixture and dextrogyros anesthetics for the cardiovascular and central nervous system. In rat sciatic nerve it showed that S75:R25 maintains the anesthetic property similar to racemic mixture (S50:R50) [21]. Two other anesthetics used for epidural anesthesia, trimecaine [C] and hexylcaine, are linked to the development of CES [22].
Spinal needles have been modified to simplify their use and minimize complications. Needle design variables, such as diameter, tip design and orifice location, have been altered to enable rapid flow of cerebral spinal fluid (CSF) and injected medications, yet simultaneously limit dural trauma and loss of CSF. The needles are classified according to their gauge and shape. A number is indicative of the needle gauge. The bigger the number, the thinner is the needle. The needles ideal for spinal anesthesia are 25G, 26G and 27G. Spinal needles in common use today are 22-27 G, but are available in sizes ranging from 19 to 30 G. By contrast, epidural needles are commonly 16-18 G. The two most commonly used needles for spinal anesthesia are the cutting point (Quincke) and the pencil point (Whitacre).
Interestingly, in the 14 patients reported in 1937 with heavy durocaine, neither type nor the caliber of the needle used was reported [19]. The vast majority of CES cases found in the literature do not report the type and gauge of the needles for either spinal anesthesia, epidural anesthesia or combined epidural-spinal anesthesia. However, in cases where needles have been described, CES can occur with both cutting-point (Quincke) and pencil-point (Whitacre) needles.
In a recent Editorial, I showed that there are several positions to perform puncture of the subarachnoid space the most common are in sitting and lateral decubitus [23]. However, there are two more positions to perform other types of spinal anesthesia, such as the prone position (Jackknife position) and in some operated patients on old orthopedic surgical tables, where there is a gap between the back and the buttocks, the puncture can be performed in the supine position with the needle entering under the table [23]. As previously stated, in the 14 patients reported in 1937 with heavy duracaine, the puncture position for spinal anesthesia was not reported [19].
With the aim of comparing the puncture position (sitting or lateral decubitus) in the different characteristics of spinal anesthesia, the study was carried out in 70 patients over 60 years old [24]. The study concluded that both sitting and lateral positions have similar effects on sensory and motor blockade and haemodynamic stability. However, patients generally found the lateral position very comfortable.
In 2009 we carried out a study of the densities of all local anesthetics and adjuvants used in spinal anesthesia with the aid of the DMA 450 densimeter [25]. The result showed that all anesthetics plus glucose are hyperbaric, while pure anesthetics (isobaric) are actually slightly hypobaric, while hypobaric anesthetics (pure diluted with distilled water) are all hypobaric. The hypobaric local anesthetic solution has not yet been commercialized in any of the countries surveyed and in Brazil, due to its little use, it has not yet been possible to launch this important solution, with important use as unilateral anesthesia and posterior anesthesia [23]. All adjuvants used in spinal anesthesia were considered hypobaric. The addition of adjuvants (hypobaric) in the same syringe, the technique used by most anesthesiologists, to hyperbaric anesthetics makes them less hyperbaric. Likewise, the addition of adjuvants to pure anesthetics (slightly hypobaric) makes them more hypobaric. And finally, the addition of adjuvants to hypobaric anesthetics makes them more hypobaric. Another way to decrease baricity is, for example, instead of producing bupivacaine at a concentration of 0.15% (p=0.99815±0.00203) for bupivacaine at a concentration of 0.1% (p=0.99726±0.00232) [26].
The vast majority of CES cases after spinal anesthesia have been with hyperbaric local anesthetic solutions (plus glucose). However, there are some cases of CES with isobaric (pure) anesthetic solutions. However, reviewing the vast majority of cases, there are no reports of CES with hypobaric local anesthetic solutions, diluted in water from isobaric ones.
Local anesthetics used in appropriate clinical concentrations and doses are innocuous drugs. Each local anaesthetic has a maximum recommended dose based on ideal body weight. However, care must be taken not to use them in high concentrations and high doses. Local anesthetic toxicity is believed to occur mainly in the cauda equina because the sacral root sheaths (a) are substantially longer (and larger for S1) than neighboring lumbar roots, (b) are devoid of protective sheaths, and (c) given their dorsal position in the thecal sac (especially L-5, S-1, and S-2), they are moreexposed to hyperbaric anesthetic pooling [27].
Studying the spinal cord of 18 dogs submitted to 1.2% hyperbaric tetracaine, 5% lidocaine, and 10% glucose, all dogs exhibited hemorrhage at the puncture site, with paralysis of the hind-legs and extensive necrosis in regions in the lumbar and sacral spinal cord. These effects are to be attributed to the concentration increase [28]. Six adult female patients underwent perineal gynecologic surgery using a spinal anesthetic of 2 ml tetracaine, 1.2%, in 10% glucose, através da lumbar puncture was performed at the L3-L4 interspace in the sitting position. The concentration of the injected tetracaine was unknown by the anesthetists. In all cases, CES was first diagnosed 72 h or later postoperatively and was confirmed in all patients.A20-yr follow-up showed that tetracaineinduced cauda equine syndrome remained stable, stressing the irreversible character of this complication [29]. The CES can occur simultaneously with vocal fold paralysis [30].
Epidural steroid therapy is a commonly used conservative therapy for lumbosacral radiculopathy. Injection of 2 mL of 0.5% bupivacaine and 60 mg of triamcinolone diacetate in 6-mL total volume causing CES that resolved within 8 hours [31].
Neural toxicity of local anesthetic and adjuvants injected into the subarachnoid space has been a matter of debate since the introduction of spinal anesthesia by Bier in clinical practice. The S-form intrathecal anesthetic drugs, especially ropivacaine, is less neurotoxic than procaine, bupivacaine, and levobupivacaine in a rat spinal model [32].
Recently, a series of 191 spinal anesthesia was published over 5 years in the same patient with a crush injury to his right leg [33]. Various anesthetics were used such as: 0.75% bupivacaine (n=151), lidocaine (n=24), procaine (n=13), mepivacaine (n=3), with fentanyl used as an adjunct 103 times, and epinephrine in 14 instances. Most of the times (n=112) the anesthetics were used pure (isobaric) and 79 times they were added with 5% glucose to transform it into a hyperbaric anesthetic. No neurological abnormalities and no postdural puncture headache (PDPH) were observed at any time. MRI scan showed some degree of subcutaneous lumbar scar tissue, whereas the epidural and intrathecal spaces showed no pathologic findings.
Experimental data suggest that ultrasound gel may cause a neural inflammatory reaction if introduced into the intrathecalspace [34]. Therefore, the gel should be used with caution when ultrasound is considered to facilitate neural puncture.
Bier was the first to use pure cocaine (isobaric) in six patients for spinal anesthesia [35]. In 1903, Braun associated hypobaric local anesthetic with adrenaline to increase the duration of anesthesia by reducing its systemic absorption [36]. In 1907, Barker introduced hyperbaric spinal anesthesia by associating stovaine with 5% glucose to increase the topographic predictability of analgesia [37].
Solutions are usually made hyperbaric by the addition of glucose in concentrations between 5 % and 8 %, and until today there is no explanation in the medical literature of this introduction and the reason for the glucose concentration used. Studying the effects of subarachnoid injection of 0.5% bupivacaine containing different concentrations of glucose (0.33%, 0.83% and 8%), showed that the maximum mean extent of block sensitivity was significantly greater with 8% glucose (T3.6) than than with 0.83% glucose (T7.2) or 0.33% glucose (T9.5), and the administration of bupivacaine containing 0.33% glucose produced a greater variability in the upper level of sensory block [38].
Effects of solutions of 0.5% amethocaine, containing different concentrations of glucose (0%, 1.25%, 2.5%, and 5%) were studied after subarachnoid injection, showed that the initial onset of effect of the solution containing 1.25 % dextrose was more like that of the isobaric solution than of those containing higher concentrations of glucose [39]. It was suggested in this study that the use of solutions containing even lower concentrations of glucose may merit investigation
Glucose is a common component of anesthetic solutions used for spinal anesthesia Studying the concentration of 10% glucose in rats suggest that, at clinically relevant concentrations, glucose does not induce neuro injurylogic, providing indirect evidence that recent clinical injuries occurring after spinal anesthesia resulted from a neurotoxic effect of the local anesthetic [40].
With the appearance of microcatheters (28G to 32G) in 1990 there was a resurgence of interest in continuous spinal anesthesia (CSA) [41]. These microcatheters are difficult to handle, the appearance CSF is slow or impossible, injection of the local anesthetic is slow, can break and provide inadequate blocks due to poor anesthetic distribution hyperbaric in the subarachnoid space, which can cause cauda equina syndrome [18,42]. In 1995, a new spinal anesthesia catheter (22G and 24G), outside needles 27G and 29G type Quincke, was used in Europe [43]. Three years after, it arrived in Brazil and one year after, the first article was published with this new catheter for CSA in 40 patients with orthopedic lower limb surgery [44]. In 2010, this catheter was used in a 78-year-old patient with massive bilateral inguinal and umbilical hernias, resulting in its safe use with large doses of 25 mg of 0.5% hyperbaric bupivacaine with 1.6% glucose associated with 160 mg of 2% hyperbaric lidocaine with 1.6% glucose, and 100 μg morphine [45].
After continuous spinal anesthesia through a 22-gauge catheter with hyperbaric bupivacaine, glucose concentrations in CSF are directly related to the highest level of sensory block, the course of the blockade, and its reversal [46].
Anesthetic records of 455 patients receiving CSA over a 17-year period were analyzed retrospectively [47]. Our resultssuggest that CSA with the catheter outside the needle for elderly orthopedic patient’s, using 0.5% isobaric bupivacaine with average doses of 8.58 mg (5 to 15 mg), shows minor insertion problem, a low incidence of hypotension, paresthesia and headache. No neurological complications were observed, such as cauda equina syndrome or transient neurological symptoms.
Spinal and epidural blocks are therefore used widely, with the more recently introduced combined spinal and epidural technique gaining popularity. Cauda Equina Syndrome results from the dysfunction of multiple sacral and lumbar nerve roots in the lumbar vertebral canal. The dysfunction of these roots can cause various symptoms, the term CES is used only when they include impairment of bladder, bowel, or sexual function, and perianal or saddle numbness. The most common cause of the CES in the general population is herniation, or bulging, ofthe intervertebral discs at the level of L4-L5 or L5-S1. It can happen in neuraxial anesthesia. Possible mechanisms include direct spine injury, intraneural injection, catheter-induced trauma, and epidural hematoma. Another postulated mechanism is poor mixing of hyperbaric local anesthetic with CSF, leading to pooling of anesthetic in the depending portion of the dural sac, especially when large volumes of anesthetics are used in continuous techniques.
The vast majority of anesthesiologists world wide use the routinely the hyperbaric solution of bupivacaine and sitting position puncture for almost all types of surgery. The use of several other forms of spinal anesthesia with isobaric, hypobaric, continuous lumbar and thoracic spinal anesthesia, combined spinal-epidural anesthesia, thoracic anesthesia, segmental spinal anesthesia should be considered and the benefits of these techniques for different types of patients and surgeries must be known [48]. Anatomy, physiology and pharmacology are very important and necessary for its understanding, and in this way apply all puncture positions, all types of anesthetic local solutions and always use the lowest dose to provide adequate anesthesia.
Having used spinal anesthesia for 48 years in the profession, luckily I have never seen this complication, with more than 300 articles on the different forms of techniques in the neuroaxial block.
Financial Support: No
Conflict of Interest: No
Contribution: No
IRB: No
This paper has not been presented.
This article was written without any financial support, being an opinion formed over 48 years of studying neuraxial blocks. Because I am a retired Professor and continue to teach anesthesiology residents, I do not have any additional salary.
