Author(s): Michael J Erquiaga, Mia R Fabbri, Michelle F Kowalczyk, Jessica E Thornton, Alyssa J Forrest, Erika L Peck, Brianna M Cunningham and J Thomas McClintock*
Decomposition is a rapidly evolving process that is dependent on various environmental factors such as climate, temperature, insect activity, large vertebrate scavenging, and microbial activity. Although other factors such as body weight and time have been evaluated, microbial activity should be considered as another major component in the decomposition process. The focus of this study the was designed to investigate the microbiome and potential bacterial succession using two different DNA extraction methods, classic microbiologic techniques and 16S ribosomal sequencing at the onset and end of decomposition. Differences were observed between the bacterial phyla found on Day 1 versus Day 6. Among the various phyla, several different bacterial species were observed such as Kurthia gibsonii, K. sibirica, Staphylococcus sciuri, S. lentus, and Serratia marcescens. An interesting change in the phyla present was observed for Day 6. None of the bacterial samples collected on any of the anatomical sites were identified in the phylum Firmicutes. In fact, most of the bacteria collected from the mouth, nose, and genitals were identified in the phyla Actinobacteria and Proteobacteria. Two different bacterial species, Myroides odoratus and Leucobacter aridicollis were present in the two phyla at Day 6 that were not observed at the onset of decomposition. Likewise, many of the bacterial species present at Day 1 were not observed in Day 6. The difference in bacterial diversity observed at the onset and end of decomposition suggest that a timeline or bacterial succession could be developed that could support post-mortem interval determinations.
Decomposition is a rapidly evolving process that is dependent on various environmental factors such as climate and temperature, insect activity and access to the body by the insects, vertebrate scavenging by raccoons and vultures, and microbial activity. Other factors such as body weight, the time elapsed, and clothing present have been evaluated as well; however, temperature is believed to be one of the most important element that can be coupled to decomposition using accumulated degree-days (ADD). To learn more about human decomposition it is critical for the forensic science community to understand the interaction of such environmental factors both with each other and with the decomposition process. Although these factors affect the decomposition process, there are certain patterns that have been shown to exist. For example, the use of insect succession to determine the ADD and the post-mortem interval (PMI) has been well-documented throughout the world in the scientific literature. To further the support for the determination and accuracy of the PMI, stages of decomposition have been used in conjunction with insect activity to determine the PMI or time of death. In particular, studies have led to the development of a decomposition scoring method to estimate the PMI and ADD [1-6].
Although some researchers have formulated their series of body decomposition stages, five discernable stages are recognized and identified consecutively as follows: the fresh stage, the bloat stage, the decay stage, the dry stage, and the skeletal stage [7]. The fresh stage begins at the time of death and includes a characteristic greenish skin discoloration (i.e., marbling), specifically in the abdominal region. The onset of bacterial activity in the fresh stage induces the bloat stage. In this stage, internal anaerobic bacteria begin to breakdown hemoglobin, digest the visceral organs, and produce gaseous by-products that attract the first insects (i.e., the flies in the families Calliphoridae and Sacrcophagidae) to the decomposing body. As gaseous by-products accumulate and create pressure in the abdominal cavity, the body is forced to distend and eventually rupture, releasing the contents into the environment. By this time, a large number of predaceous coleopterans (i.e., beetles in the families Silphidae and Staphylinidae) begin to arrive at the corpse to feed on the dipteran or fly larvae. At the end of the third stage, the decay stage, most of the flesh has been removed from the corpse and most of the insect and microbial activity has ceased. The final two stages of human decomposition, the dry and skeletal stages, leads to the reduction of the corpse to only leathery, shrunken and dark skin, cartilage, and bone.
As insect activity produces a predictable pattern, microbial activity, especially bacteria, should be considered as another crucial component in the decomposition process. Previous studies investigating the microbiome during decomposition have attempted to identify the numerous varieties of bacteria present on the decaying body using either classic microbiological techniques or terminal restriction fragment length polymorphism. Recent studies have used high throughput sequencing techniques to identify the microbiome in a decomposing body [8-10]. The focus of this study was designed to investigate the microbiome and bacterial succession, using both classic microbiological techniques and 16S ribosomal sequencing to determine specific bacterial prevalence at the onset and end of decomposition. The use of bacterial succession and shifts in community structure during decomposition could potentially be used in criminal investigations, along with insect succession, to determine the PMI.
Two local, fresh cadaver pigs (approximately 40 lbs) were placed in separate cages at Liberty University s outdoor research facility to decompose under natural conditions. Bacterial samples were collected at the onset of death (i.e., Day 1 or time zero) and then again at the end of decomposition (i.e., Day 6). Various anatomical structures were swabbed: mouth, ears, cheeks, nose, and genitals. Swabs, containing the samples, were placed in separate paper wrappings to avoid cross-contamination, placed in a storage container, and returned to the laboratory for analysis.
Bacterial samples from the swabs were cultured on nutrient agar and/or R2A agar (Reasoner s Agar similar to minimal media) and streaked for isolation. In some instances, serial dilutions of the samples were necessary due to the number of bacterial cells present. Following isolation, individual colonies were Gram stained, the cell morphology determined, and then subjected to standard biochemical tests (e.g., catalase, oxidase, nitrate reduction, urease, etc.) used for identification purposes. Gramnegative bacillus-shaped bacteria that were catalase-positive were identified using the EnteroPluri-Test (Becton, Dickerson and Company, Sparks, MD), a rapid diagnostic assay that tests for the presence of bacterial members in the family Enterobacteriaceae.
DNA was manually extracted and purified from isolated bacterial colonies by using either a freeze-thaw method (i.e., three cycles at -70°C for 3 min followed by 100°C for 2 min, centrifuged at 10,000g for 5 min, and the supernatant collected in a microcentrifuge tube) or by robotic extraction (AutoMate Express Nucleic Acid Extraction System with Prep-Filer lysis buffer, Thermo Fisher Scientific Inc., Waltham, MA). The extracted DNA, from both methods, was quantitated using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA) and analyzed by agarose gel electrophoresis. Approximately 10 ng of bacterial DNA from each isolate was amplified with the primers 8F (5 - AGA GTT TGA TCC TGG CTC AG - 3 ) and 1492R (5 - GGT TAC CTT GTT ACG ACT T - 3 ) following the methods outlined by Lauer et al. This primer pair is capable of amplifying most of the hypervariable regions in the ribosomal DNA (rDNA) gene, and thus able to identify a wide variety of bacterial taxa [11,12]. Reactions were heated at 95°C for 4 min followed by 34 cycles at 94°C for 1 min at, 53°C for 1 min, 72°C for 90 sec, and 1 cycle at 72°C for 10 min. PCR amplicons were analyzed on a 1% agarose gel and visually confirmed before sequencing. Samples were subjected to Sanger sequencing at Eurofins Scientific (Louisville, KY).
Data Analysis
A consensus sequence for each isolate was obtained by aligning the sequences of the forward and reverse amplicons. 16S rRNA gene sequencing data were analyzed using BLAST, and the sequences of these isolates were compared to the National Center for Biotechnology Information (NCBI) 16S rRNA (Bacteria and Archaea) database [13]. The sequencing and taxonomy results were then compared to the EZ Biocloud (https://www. ezbiocloud.net/resources/16s_download) database. Reads were quality-filtered and trimmed to 1000 - 1100 bp and binned into operational taxonomic units with a sequence identity of 97%. Identification to the family level, or even to the genus and species level, was assigned using the closest reference sequence match based upon the sequence similarity. An overview of the entire isolation, extraction, amplification, and 16S ribosomal sequencing process is presented in Figure 1.
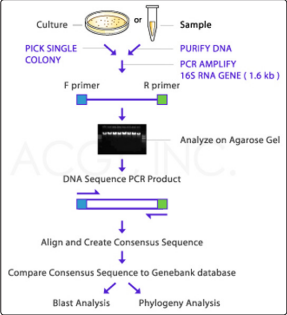
Figure 1: An overview of the isolation, extraction, amplification, and 16S ribosomal sequencing process (Courtesy: ACGT, Inc., Germantown, MD).
This study was designed to investigate the microbiome and potential bacterial succession at the onset and end of decomposition. Initially, bacterial identification was performed using classic microbiological techniques that included biochemical testing, the EnteroPluri-Test for Gram-negative and catalase positive bacilli, as well as the BIOLOG bacterial identification system (Hayward, CA). With the exception of the BIOLOG system, each method allowed for the identification of bacterial isolates which could then be used for comparison to the sequencing results. Although promoted as a rapid identification system, we were unable to successfully utilize the BIOLOG system in a manner that would yield reproducible and reliable results. However, due to the numerous bacterial species collected at each anatomical site and the amount of time needed for identification, these methods were relinquished for 16S rRNA gene sequencing and bacterial identification.
Two different DNA extraction methods were utilized and compared: a freeze-thaw method and an automated robotic system (AutoMate Express), both of which yielded comparable results (Figure 2). Though the use of both methods and subsequent analysis by gel electrophoresis, both manual and robotic extraction were found to be effective for extracting bacterial DNA. Furthermore, for each of the two methods, 3-4 samples consistently did not appear to yield isolated DNA. In the case of manual extraction, these samples did not fully form a pellet following centrifugation, and thus the supernatant, containing the DNA, also harbored extraneous cellular material. In the case of the AutoMate-extracted sample group, the non-isolated samples contained remnants of beads used to separate the DNA from the cellular materials. Although DNA banding was not always visible following gel electrophoresis, amplified products were observed after PCR amplification. However, it should be noted that while both extraction methods produced reliable yields the robotic AutoMate system provided a quicker turn-around time and a more consistent DNA product.
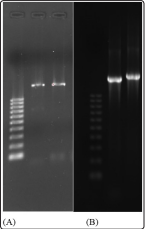
Figure 2: (A) DNA was extracted manually from bacterial isolates, amplified by PCR, and analyzed by agarose gel electrophoresis. (B) AutoMate-extracted DNA was amplified by PCR and the products analyzed by agarose gel electrophoresis. Far left lane for both panels is the 1 kb DNA ladder with ten 100 bp fragments.
Differences were observed between the bacterial phyla found on Day 1 (Proteobacteria, Actinobacteria, and Firmicutes; Figure 3) versus Day 6 (Proteobacteria and Actinobacteria; Figure 4)

Figure 3: Relative abundance of Phylum Present at Day 1.

Figure 4: Relative abundance of Phylum Present at Day 6.
On Day 1, the samples collected from the cheek and ear contained bacteria predominantly in the phylum Firmicutes; whereas samples collected from the mouth and nose contained bacteria that were in the phyla Actinobacteria and Proteobacteria. The most abundant bacteria that were classified in the phylum Proteobacteria were collected from the genitals. Throughout the various phyla, several different bacterial species were observed such as Kurthia gibsonii, K. sibirica, Staphylococcus sciuri, S. lentus, and Serratia marcescens (Table 1). These bacteria, commonly found in the environment, are also consistent with the normal animal and/ or human microbiome. Additionally, data collected for Day 2 revealed a different population of bacterial species that included Micrococcus lylae, M. albus, Klebsiella oxytoca, Marinococcus albus, and Staphylococcus lentus (data not shown).
Table 1: Variations in bacterial growth between the different anatomical regions of the bodies on Day 1| Mouth | Nose | check | Ear | Genitals | |||||
|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus sciuri | 20% | Staphylococcus sciuri | 20% | Staphylococcus sciuri | 67% | Staphylococcus sciuri | 57% | Luteimonas padinae | 43% |
| Brachybacterium massiliense | 20% | Brevundimonas olei | 20% | Glutamicibacterer mysorens | 33% | Lysobacter defluvii | 28% | Corynbacterium freneyi | 14% |
| Brevundimonas naejang sanensis | 20% | Arthrobacter koreensis | 20% | -- | -- | Glutamici creatinolyticus | 14% | Alcaligenes faecalis subsp. phenolicus | 14% |
| Luteimonas padinae | 20% | Lysobacter spongiicola | 20% | -- | -- | -- | -- | Lysobacter defluvii | 14% |
| Brevibacterium avium | 20% | Serratia marcescens | 20% | -- | -- | -- | -- | Staphylococcus lentus | 14% |
An interesting change in the phyla present was observed for Day 6. None of the bacterial samples collected on any of the anatomical sites were identified in the phylum Firmicutes, but rather, the majority of bacteria collected from the mouth, nose, and genitals were identified in the phyla Actinobacteria and Proteobacteria. When looking at the bacterial species found within these two phyla at Day 6, two different bacterial species, Myroides odoratus and Leucobacter aridicollis were present that were not previously observed at the onset of decomposition. Likewise, many of the bacterial species present on Day 1 were not observed on Day 6. It should be noted that since only two days were used for samples in this data analysis, a progression cannot be shown, but the difference in the diversity of bacteria collected at the onset and end of decomposition indicates that a timeline or bacterial succession could be developed, provided data for the intermediate days be included in future studies. Not only were trends found within the types of bacteria found to be either present or absent across the time points, but the type of bacteria present may also be dependent upon the anatomical region of the body from which the sample was collected.
As with any technique, there are advantages as well as disadvantages, and the 16S rRNA gene sequencing procedure is no exception. The sequencing procedure is relatively inexpensive and is designed for high throughput, allowing for the analysis of many samples. Moreover, these well-developed analytical tools paired with reference databases allows the researcher to identify microorganisms not only to the family and genus level but down to the species level. Some of the disadvantages may not seem obvious at first. For example, many more bacterial cells are observed under the microscope than grown under standard laboratory conditions. Thus, culture-independent methods are needed to examine the unculturable majority of bacteria in a sample. Also, the information that is gathered only provides some insight into the relative proportions of bacterial taxa; hence, this method is qualitative, not quantitative, providing a compositional image of the bacteria present at a given time.
The methods for DNA extraction used in this study resulted in very pure DNA for sequencing, and thus, it can be concluded that while classical DNA extraction techniques can be conducted, the automated system used in this study provided much more consistent yields at an expedited rate. The sequencing data acquired following DNA extraction, purification, and amplification, revealed a very diverse group of bacteria, not only for each anatomical region sampled but also for both days examined in the decomposition process. Although the sequencing procedure and data have some limitations this method overcomes culturing bias and is relatively inexpensive and accessible.
Due to the similarity between the microbial activity on the decomposing cadaver at Day 1 and the human microbiome, it could be suggested that bacterial prevalence or activity during human decomposition follows a trend much like that of the surrogate pigs used in this study. If so, this would further suggest that the bacteria identified from Days 1 and 6 during decomposition could be parallel to bacteria succession associated with human decomposition, thus paving the way for a potential determination of the PMI for humans. The difference in bacterial diversity observed at the onset and end of decomposition indicates that a timeline or bacterial succession could be developed, should data for the intermediate days be incorporated into future studies, which could support PMI determinations. Though there is much left to be determined in this specific area of forensic sciences, the results of this study provide a clear basis for continued exploration.
