Author(s): Hossam B Bahnasy
Diabetes mellitus (DM) is a metabolic chronic disease that represents a high global incidence rate. Glycated hemoglobin (HbA1C) is the long - term glucose reference test it appears to be associated with chronic diabetes complications, (Hb A1C) is not recommended for use in clinical situations that may interfere with metabolism of hemoglobin, as in hemolytic anemia, secondary or iron deficiency, hemoglobinopathies, pregnancy, and uremia.Glycated albumin (GA)is ashort term blood sugar test that is not affected by cases that incorrectly change A1c leveles.GA is the top part of glucose of fructosamine. It is meashured by the standard enzyme methodology, easy and fast performance.These Laboratory properties have ensured the spotlight on GA in studies from the past decade,as asign of monitoring and screening for diabetes as well as predicting long –term outcomes of the disease . The aim of this review was to discuss the physiological and biochemistry characteristics of the GA, as well as its clinical utility in DM.
Diabetes mellitus (DM) is a chronic metabolic disease, which is caused by the decrease of insulin secretion and even by the decrease of insulin sensitivity. Currently,DM it is aglobal epidemic and amajor challenge to health care systems everywhere .The international Diabetes Federation (IDF)estimates that one in 11 adults has DM , which has atotal population of about 415 million , and 193 million of them have not done so after being diagnosed. Chronic hyperglycemia is a common feature in all subtypes of this disease and is associated with long-term damage, which increases the morbidity and mortality rates and causes dysfunction of different organs, such as blindness, kidney failure and amputation. These chronic complications are expensive for healthcare systems and decrease life expectancy for diabetics. Currently, laboratory tests are used to diagnose diabetes are glycated hemoglobin (A1C), fasting blood glucose (FG) and two-hour blood glucose (2hG) after a 75g oral glucose tolerance test (OGTT). A1C is also the benchmark test for blood sugar monitored because it directly reflects average blood sugar and is strongly associated with long-term complications of diabetes. However, A1C is not recommended for use in some clinical conditions affecting hemoglobin metabolism [1-5], because potential interference with its results, rendering it misinterpreted. Furthermore, recent studies have shown a that there is a difference between A1C levels in different ethnic groups at equal levels of glycemia, but the reasons for these discrepancies have not been well explained so far. Glycated albumin (GA) is a laboratory test that has acquired some importance to monitor blood sugar in DM in past decades. GA is one of the fructosamines, but it has the advantage of not being affected by other serum concentration proteins because they are specific to the sugar albumin levels. Moreover, GA does not require fasting to measure and reflects short-term blood sugar due to the life span of albumin is about 3 weeks. Compared with A1C, GA was not affected by the presence of hemolysis and abnormal Hb. Besides, in cases such as anemia, pregnancy, postprandial hyperglycemia and DM using insulin, GA appears to be a better blood sugar mark than A1C and is also particularly recommended for diabetics undergoing dialysis. In recent years, studies on patients with type 1 and type 2 diabetes have shown the relationship between GA and chronic disease complications. Although GA has been studied in the last few years, this test has not been widely used in lab routine, and few commercial reagents available on the market for its analysis. However, the results of clinical studies make GA a promising marker of DM. Under this background, this review puts forward some suggestions on the physiological and laboratory characteristics of GA, and discuss its clinical application in the diagnosis and management of DM [8-17].
Albumin is a protein with high molecular weight with 66.7 kDa, consisting of a single polypeptide chain containing 585 amino acids, 17 bridges of disulfide and 3 homologous domains that are connected in a helical structure [18]. It is the major plasma protein, and it represents about 60% of the total protein in the blood, with concentrations between 3.0 and 5.0 g/dL and a half-life from14 to 20 days. The structure of albumin facilitates the performance of its physiological functions, such as maintaining pH and osmotic blood pressure. Also, albumin acts as a powerful antioxidant and as the main transporter of metabolic products, ions, nutrients,drugs, hormones and fatty acids. Like other proteins, albumin also through the physiologic process of glycation. By definition, the glycation is a non-enzymatic autoreaction in which reduced sugar is added to a free amino group, usually lysine or arginine found within proteins, also called the Maillard reaction (Figure 1). The first step of this reaction involves the formation of an unstable and reversible product known as Schiff base, formed by the bonding of a carbonyl group of an acyclic carbohydrate with the N-terminal amino acid. This intermediate product can suffer a change in its shape and results in stable and irreversible ketamine, known as the Amadori product. The main adductor made up of amadora is fructoselysine, the interaction between glucose and lysine, which may occur at the 59 sites of lysine exist in albumin. However, lysine 525 has been identified as the greatest sugar in albumin site, which has been proven in both in vivo and in vitro experiments. A group of ketamines which consists of non-enzymatic glycation of proteins is chemically called “fructosamine”. Among the fructosamines in the blood, GA is the main component, it accounts for about 80% of the total sugar in the plasma [18-24].
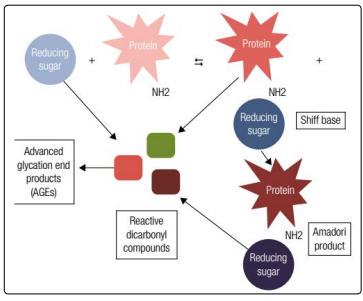
Figure 1: Maillard reaction illustration. In the first glycation stage, there is production of Shiff base by a reaction between a reducing sugar and a free amine group present into the polypeptide chain of plasma proteins and, subsequently, a rearrangement yield the Amadori product. In the next steps, the degradation of the Shiff base and Amadori products, as well as the oxidation of sugar responsible for the formation of interactive dicarbonyl compounds, known as AGEs’ precursors
The glucose concentration and time of exposure between protein and sugar are the determining factors for the glycations performed during the life of the protein. In other words, glycation depends on the degree and duration of hyperglycemia (high blood sugar) [22]. Extracellular proteins, such as albumin, may be more easily to Amadori rearrangements than intracellular proteins such as Hb [18]. This is due to plasma proteins direct exposure to plasma glucose. These characteristics can justify the differences in the levels of albumin glycation that are about 9 to 10 times higher than those found in hemoglobin [25]. However, in laboratory experiments conducted by Ueda and Matsumoto, it was also proven that GA production was about 4.5 times greater than A1C after adding known and equal concentrations of glucose in previously treated samples of healthy volunteers. These results showed that even under symmetric glucose conditions, GA is produced faster than A1C [20].
Inthe advanced stage, of glycation, additional oxidative and irreversible events occur regarding the glycated proteins, producing stable and heterogeneous compounds known as advanced glycation end products (AGEs - Figure 1) .Although AGEs configuration is a normal process, and the typical hyperglycemic conditions in DM patients increase their production rates. AGEs receptors are present in cells of different tissues, such as macrophages, muscle, endothelial and glial cells. They are expressed as membrane molecules, a necessary part of immunoglobulin superfamily and theyact as receptors for signal transduction, which lead to oxidative stress and start-up an inflammatory cascade by activation of nuclear factor-kB (NF-kB). NF-kB regulates the gene transcription of proinflammatory molecules such as interleukins 1, 6 and 8 and tumor necrosis factor-α, as well as the vascular cell adhesion molecule-1 and intercellular adhesion molecule-1[26,27]. As a result of this cascade, there is an increased production of reactive oxygen species, which are directly associated with the pathogenesis and long-term complications of diabetes [21,27]. Kisugi and cols. evaluated samples from adiabetic patient within one month of hospitalization due to symptoms of hyperglycemia and evidenced that the formation of AGEs was drastically reduced with the accompanying decrease in GA levels [24].
Historically, fructosamine was used in clinical practice when a short-term glycemia evaluation is needed. However, this test provides a low accuracy since it is affected by all plasma proteins as well as other molecules present in the blood, such as bilirubin, uric acid and low molecular weight substances [12,28]. In addition to, fructosamine is not available in all laboratories [18,29] and there is no well-established international standards for use. GA evaluation Methods have been developed since the 1980s using serum or plasma samples [28]. The olde methods presented many disadvantages due to the complexity of the techniques or the high costs and/or lack of precision. In addition, the non-standardization of these assays reinforce the unpopularity of GA, and all attention were directed to A1C [30]. GA can be evaluated by ion-exchange high-performance liquid chromatography (HPLC), boronate affinity chromatography, immunoassays (radioimmunoassay and Enzyme Linked Immuno Sorbent Assay), colorimetric measurement method with thiobarbituric acid and enzymatic methods using proteinase and ketamine oxidase [12, 28, 29, 31], these methods are not currently available in laboratory routines [32].
The reference interval are used to describe GA depends on the method used since GA levels may differ according to the glycation sites analyzed by the assay used, and also if the analysis method is considered the GA molecule for measurement and not its glycated amino acids [31]. For example, immunoassay techniques, colorimetric methods with thiobarbituric acid and enzymatic methods take into account the glycated amino acids as the reference for the GA levels. On the other hand, HPLC techniques and other chromatography methods consider the GA molecule to determine their levels. In spite of this difference, all available methods agree that the proportion of GA in patients with DM increases 2 to 5 fold compared to normoglycemic patients [18].
An enzymatic methodology with a shorter runtime and easier to perform both manually and automatically to evaluate the GA levels in order to overcome the limitations of the pre- existing techniques. This method provides three steps (Figure 2), procedure using specific proteinase for albumin and ketamine oxidase, as well as the bromocresol green reagent to evaluate albumin and later calculation of %GA. In the verificationprocess made to introduce the test to the market, the analytical performance was excellentand the assay was not influenced by bilirubin and glucose, but a slight interference in the GA levels in the presence of Hb and ascorbic acid was reported [12]. Other studies described similar results, concluding that the new enzymatic methodology, known as “Lucica GA-L®” (Asahi Kasei Pharma Corporation, Tokyo, Japan) showed reproducibility, accuracy and a good correlation with A1C [30, 31]. Subsequently, other manufacturers have released similarly methodologies for GA analysis, but instead of a specific measurement of its levels, these assays employ math equations to obtain %GA levels. In addition, the biological variation of GA measured by Lucica GA-L® is lower when compared to fructosamine and A1C (1.7%, 2.8% and, 2.4%, respectively) [33-35].
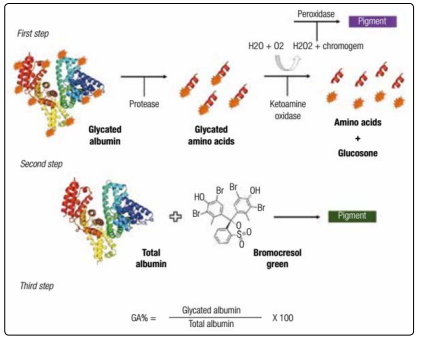
Figure 2: Enzymatic reaction for GA determination. First step: release of the glycated amino acids from the GA molecule through an albumin-specific protease. A ketoamine oxidase segregate the free amino acids and glucosone, this is the last intermediate product of Amadori reaction. The final pigment is proportional to the amount of GA in the sample; The second step: is the reaction of plasma albumin with bromocresol green in an acidic medium, and the resulting coloring compound is related to the total albumin concentration; Third step: the percentage of GA is obtained by a mathematical calculation given the two previous reactions.
GA offers good stability when frozen at very low temperatures. In the study of the Kohzuma and cols. samples frozen at -80°C maintained a stable GA levels for 4 years [31]. Watano and cols. similar results were found for storing serum samples at -70°C. However, they observed a considerable increase in GA levels frozen at -20°C after 6 months [36]. Nathan and cols. measure GA levels in samples of participants in the study The Diabetes Control and Complications Trial Research Group (DCCT) frozen at -70°C 23 years ago and concluded that the stability of this analyte stayed adequate [16].
However, despite all the mentioned characteristics, GA testing is not yet available regularly in laboratory practice, but it has been used frequently in DM clinical research in the last decade [3]. The factor responsible for the increased number of studies on GA was the consolidation, although without a defined international consensus, of Lucica GA-L® enzymatic assay for GA determination.
In clinical practice, A1C is used as a reference test to monitor glucose in DM, and it is also a diagnostic tool [2]. However, there are some specific disadvantages and argument that limit it use. They are related to certain clinical situations or to the analytical methods employed [3,6]. These conditions may yield false results for A1C that are not truly correlated with the mean glycemia [28], and directly affecting the identification and management of patients with DM. In such cases, GA may be an adequate alternative for A1C in the glycemic control [37].
GA can be used as a substitute for A1C in any blood change interfering in the half-life of red blood cells and/or in the composition or chemical properties of Hb [28]. Hemolytic anemias and bleeding episodes reduce the A1C values, while iron deficiency anemias, thalassemias, and hemoglobinopathies may elevate its results [6,11,38]. In the fetal period, the main type of hemoglobin in the red blood cells (RBCs) is the fetal Hb (HbF), which is gradually substituted by HbA after birth. Since A1C is a glycation product of the HbA, and newborns tend to have falsely lower levels of it [39]. However, the overlaps with A1C measurements depend on the method. Some analytical methodologies may not be affected by common interventions such as hemoglobin variables. The National Glycohemoglobin Standardization Program (NGSP) provides detailed information on interference in A1C testes by manufacturers [7].
During pregnancy, it is recommended that women who already have diabetes and those who develop gestational DM are advised followed by self-monitoring of glucose and A1C levels [2]. However, it has been proven during the last months of pregnancy there is an increased demand for iron, which is directly reflected in the changes in the A1C throughout the pregnancy. In a prospective study by Hashimoto and cols. performed on pregnant Japanese women with DM, they had a significant elevation in A1C it is found at the end of pregnancy, inversely to the ferritin levels and transferrin saturation. On the other hand, GA remained stable during this period, because it was also not experiencing interference from the physiological changes characteristic of pregnancy [40,41].
In DM and CKD patients, A1C may not be a reliable marker of blood sugar control. Patients with CKD generally suffer from erythropoietin deficiency and, therefore, develop anemia. Thus it is necessary to use exogenous erythropoietin to compensate the diminished endogenous synthesis by the kidney, and also iron, which falsely alters the levels of A1C. Moreover, these patients may require blood transfusion often times , when dialysis is performed, their life span of erythrocyte is shorted by20-50% , which also contributes to false A1C values [42,43]. Increased uremia in CKD results in the production of carbamylated Hb, which is an interfering agent in vitro in some analytical methodologies for A1C [6]. Some studies have shown that GA provides a more accurate control of blood sugar levels in patients with advanced stages of CKD [14,15,42]. However, in the presence of massive proteinuria with diminished serum albumin, the GA levels can also be falsely altered [42,44], and it is necessary to perform a critical evaluation and sufficiently choose the best glycemic marker in this condition.
Despite the evident informative value of A1C in monitoring DM, some authors have questioned the cutoff point used for this test in diagnosing the disease. This is because the current approved criteria show a discrepancy between the ratio and profile of patientsidentified as having DM by the A1C, compared to the glycemia based tests [45,46]. Additionally, patients with special conditions that interfere with A1C results should be screened for DM using alternative markers. Since the enzymatic method for GA has been developed recently, few diagnostic accuracy studies GA has been published for DM (Table 1).
| Study | N | Country | Male | RI GA (%) | DM cutoff | SxS |
|---|---|---|---|---|---|---|
| Tominaga and cols. 2006 | 699 | Japan | 52% | 12.3 - 16.9 | - | - |
| Paroni and cols. 2007 | 32 | Italy | 37% | 11.7 - 16.9 | - | - |
| Kohzuma and cols. 2011 | 201 | USA | 47% | 11.9 - 15.8 | - | - |
| Furusyo and cols. 2011 | 1.575 | Japan | 30% | 12.2 - 16.5 | 15.5% | 83.3 x 83.3 |
| Hwang and cols. 2014 | 852 | Korean | 58% | - | 14.3% | 66.4 x 52.5 |
| Ikezaki and cols. 2015 | 176 | Japan | 46% | - | 15.2% | 62.1 x 61.9 |
| Hsu and cols. 2015 | 2.192 | Taiwan | 50% | - | 14.9% | 78.5 x 80.0 |
| Testa and cols. 2017 | 252 | Italy | 38% | 9.0 - 16.0 | - | - |
RI GA: reference interval for GA; SxS: sensitivity and specificity to the cutoff points found.
In 2006, the Japan Diabetes Society (JDS) has established a reference interval for GA from 12.3% to 16.9%. Years later, in a larger study (N = 1.575), Furusyo and cols. published a GA reference interval of 12.2% to 16.5%, corroborating with that reported by the JDS. Moreover, this study found that the GA cutoff point ≥ 15.5% showed a good sensitivity and specificity (both 83.3%) for determining DM, using FG and/or A1C (≥ 126 mg/dL and ≥ 6.5%, respectively) as reference tests. In 2015, the same group evaluated 176 Japanese residents who had previously been diagnosed with DM by the OGTT, according to WHO criteria. ROC curve analysis showed that GA made significant differences in the area under the curve (AUC)to diagnose DM, and these defferences increased values when combining GA with FG or 2hG than GA at isolattion (AUC: 0.863, 0.968 and 0.672, respectively) [47-49].
Hwang and cols. assessed different cutoff points of GA for DM and pre-DM diagnosis in 852 Korean adults, using the ADA criteria to classify the disease. The study reported a cutoff point of 12.5% for pre-DM and 14.3% for DM. GA presented greater sensitivity than A1C (66.4% GA versus 52.5% A1C), but less specificity (88.3% GA versus 95.1% A1C) to predict 2 hG ≥ 200 mg/dL. When the values of 14.3% of GA were associated with FG ≥ 126 mg/dL, higher sensitivity was obtained (77.5%, CI: 72.17-82.0) to diagnose DM. Hsu and cols. described a cutoff point of GA ≥ 14.9% for DM (sensitivity: 78.5%; specificity: 80.0%), evaluating 2,192 adult individuals in Taiwan. Also, when the values were 5.7% and 6.5% of A1C in consideration, the corresponding GA was 14.5% and 16.5%, respectively [50,51].
Studies describe GA intervals in individuals without diabetes ranging from 11.9 to 15.8% (N = 201 residents of North Carolina, USA) [31]; 10.2 to 16.1% (N = 217 African immigrants in America) [52]; 10.5 to 17.5% (N = 44 volunteers of a Canadian study) [33]; and 9.0 to 16.0% (N = 252 European persons) [53]. In young obese persons aged from 10 to 18 years, the value of GA to diagnose DM was ≥ 12% when 2 hG was used as the reference test, and ≥ 14% when A1C was used as reference diagnostic criterion [54].
Differently from A1C long-term formation (about 120 days, mean life of the erythrocytes), GA is formed in a period of approximately 2 to 4 weeks (Figure 3) [37]. This feature enhances GA sensitivity to the rapid alterations in glucose levels, which may not be efficiently identified with an isolated measure of plasma glucose [13,19].
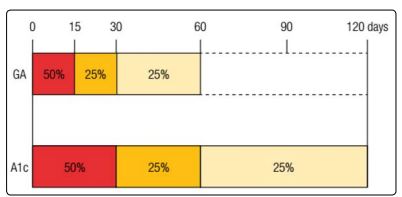
Figure 3: Glycation rates of GA e A1C. GA is produced over the shelf life of albumin about 8 weeks, however, the first two weeks account for half of his production. Differently, due to the life span of erythrocytes, that is around 120 days, A1C takes approximately 4 months to be completely produced, and the first month is responsible for half of its glycation.
Compared with A1C, GA is more suitable for monitoring the onset of drug therapy in the DM [55], as well as to control the dose and change of medication [51], since levels are diminished faster than A1C in intensive treatment [19]. Paroni and cols. Proved that GA was a better marker for assessing the responses to treatment with insulin in type 2 DM patients with insufficient glycemic control, and also that GA presented a greater correlation with FG than A1C (R = 0.75 versus R = 0.54, respectively) [30]. Moreover, Yoon and cols.It reported that the deterioration of pancreatic beta cell function was associated with the time of duration of DM, and also increased GA and GA/A1C ratio, but not with A1C alone [56].
In general, GA can be used to show average blood sugar and also to assess the glycemic fluctuation and postprandial glucose levels more appropriate than A1C [37,57]. Increases in postprandial glycemia are associated with the increased risk of cardiovascular diseases and microangiopathy, thus detecting these important of glucose differences [37]. Reasons why GA is more related to postprandial glycemia have not yet been clarified [11].
Chronic hyperglycemia significantly increases the risk of developing micro and macrovascular diseases over time [1,2]. A1C is a marker that has been strongly discovered in clinical research and has supported much evidence of its use as an predictor for these complications in DM [4,5]. However, there is still debate if it means glycemia itself or glycemic fluctuation is the maindetermining factor for chronic damage in DM [16]. Recent studies have assessed the predictive potential value of tests that are more related to short term glycemia and which can be used as alternative markers for A1C, such as GA [8,16,17,58].
Selvin and cols. The review assessed 1,600 individuals were recruited for Atherosclerosis Risk in Communities (ARIC) study conducted in the USA. They note that in participants with type 2 DM, it was both GA and fructosamine were significantly associated with the albuminuria, CKD, and retinopathy [8]. In a longitudinal study, the same group assessed 12,306 ARIC participants, who it has been followed for over 20 years and proven to be both GA and fructosamine they were similarly correlated with A1C to predict retinopathy and CKD in DM. These results were confirmed in patients diagnosed with DM during the baseline period and in those who developed diabetes during the follow-up. Odds ratio (OR) observed the occurrence of retinopathy in patients with DM and GA levels between 15.7% and 23.0%, was lower than when GA > 23.0% (OR > 8 and OR > 15, respectively), even in a statistical model adjusted for the A1C levels [17]. In addition, Selvin and cols. have also showed a similar association between GA and A1C with regard coronary heart disease, ischemic stroke, heart failure, and death [59].
Nathan and cols. data used from DCCT and Diabetes epidemiology Interventions and Complications (EDIC) studies to assess the correlation between GA and chronic complications in type 1 DM. They demonstrated that GA, as well as A1C, were strongly associated with the emergence of retinopathy and nephropathy after an average follow-up time of 6.5 years, but no evidence was seen on the 7-points glucose profile. Only A1C was associated with cardiovascular disease [16]. In another study with 154 type 1 diabetic patients they followed over 2.8 years, developing to nephropathy was associated with only GA and not A1C. The authors found no link between these two glycemic markers and the cardiovascular outcomes [58]. Apparently, GA is a predictor of microvascular complications in type 1 and type 2 DM. However, with regard to macrovascular results, GA appears to be a good marker only in type 2 DM. The mechanisms involved in the development of atherosclerosis and cardiovascular diseases in type 1 DM might explain these findings.
Some situations that interfere with albumin metabolism may also influence GA values. In theory, GA is not changed by albumin levels in the blood, as its values are also corrected for total albumin, but low levels of this protein are associated with increased glycation rates. On the other hand, increased protein metabolism implicates in lower GA levels [60]. Therefore, in cases of hyperthyroidism, hypothyroidism, liver cirrhosis, nephrotic syndrome with huge proteinuria, or other specific disorders, GA uses may be misleading and should be avoided [32]. However, because this test is relatively new, few studies have been carried out to verify interfering factors in GA levels. Other interfering situations on GA levels already described are age, obesity and inflammatory conditions (observed by the increase of C-reactive protein), smoking, and hypertriglyceridemia [11,32,37]. There is little evidence for an explanation from GA in different ethnic groups. However, Selvin and cols. It analyzed 1,376 people without DM and 343 with DM, and found that both GA and A1C were significantly higher in Blacks compared to Whites [8]. Thus, the data presented here demonstrated the need to be careful when interpreting GA levels in some clinical cases.
GA is a short-term glycemic marker that was evaluated as an alternative test to A1C in DM patients. If compared to A1C, GA evaluation is more reliable to glycemic fluctuation. It is also indicated especially for patients undergoing dialysis and its levels are not affected in the presence of anemia or hemolytic processes. Compared to the fructosamine test, GA is more helpful, as it is not affected by other serum proteins. The enzymatic methodology for its analysis is easy and fast implementation, and analytically highly efficient and with greater standardization. Such as described previously, in clinical situations that incorrectly alter A1C levels, GA measurement may determine areliable result of DM monitoring. However, the formation physiology of these two glycated proteins ensures the advantages of GA compared to A1C in access glucose control, even in the absence of overlapping factors. Finally, several studies have shown that GA has good diagnostic accuracy and is closely associated with microvascular complications of diabetes. Although all the benefits from GA, it does not replace the use of A1C, once each test has its advantages and limitations. The choice on which test should be used by the clinical availability of patient features and tests should be guided. Moreover, it is necessary internationally consensus on laboratory issues and clinical use of GA, to ensure their inclusion in the routine of clinical laboratories worldwide, thus improving future screening and management of DM patients.
