Author(s): Jeevan S Ladi*, Tanmayi Dhamankar, Shalu Chavan, Nitant Shah
Purpose: The Barrett’s formula requires optical biometry inputs. The Ladi adjustment was designed to overcome this and use ultrasound (US) axial length (AXL) inputs into Barrett’s universal II formula. We compared the residual refractive error (RRE) with optical vs. ultrasound biometry with Ladi adjustment following phacoemulsification when all IOL powers were calculated using Barrett’s formula.
Study Design: Prospective, randomised, double blinded study
Methods: All adults undergoing routine phacoemulsification were recruited. The keratometry and AXL were randomly measured using either the IOL master 500 (IM500) or a combination of US biometry and Sirius topography. For the US measurements, 200 microns was added to the AXL (Ladi adjustment) and values were entered in the online Barrett’s calculator. The RRE (spherical equivalent – target refraction) was determined at 6 months.
Results: We included 200 eyes of which 100 had IOL-power calculation using the IM500 and 100 using the Ladi adjustment of the US. The groups were comparable in baseline characteristics. The RRE was comparable in the two groups (-0.09+0.27D in US vs. -0.08+0.23D in IM500, p=0.90). The RRE was slightly higher in eyes with AXL>25mm (n=18 eyes, -0.21+0.33D in US vs. -0.07+0.17D in IM500), though this was not statistically significant (p=0.27). The preop BCVA (β= 0.06D, 95%CI= -0.5 to 0.13D, p=0.07) was the only factor marginally influencing RRE.
Conclusion: The US biometry with the Ladi adjustment was as accurate as the IM500 and can be reliably used with the Barrett’s formula for IOL power calculations. Caution should be maintained at extremes of AXL.
Cataract surgery has improved immensely such that it is now considered as a refractive surgery and patients expect to be spectacle free postoperatively [1,2]. The introduction of premium intraocular lenses (IOLs) has played a major role in this evolution over the past decade. In addition to the tried and tested monofocal IOL, multifocal, toric and extended depth of focus (EDOF) IOLs have enabled surgeons cater to the individual visual needs of their patients[2-4]. Ocular biometry has also improved significantly from ultrasound technology to optical methods using partial coherence interferometry, optical low-coherence reflectometry and more recently, swept-source OCT-based machines that are accurate even in dense cataracts [5-8].
All these advancements are brought together by IOL power calculating formulae which have also seen paradigm shifts over the last decade. The accuracy of these formulae has improved significantly and newer formulae incorporating artificial intelligence promise to improve accuracy further [9-12]. Majority of research in the past few years have shown that the Barrett’s universal II formula is perhaps the best one for all axial lengths and is being used most widely [9,10]. However, most of these newer and advanced formulae require axial length measurements using optical biometry. Recent reviews on the subject don’t even mention the ultrasound technique when discussing advanced formulae [5]. These machines are expensive to purchase and maintain and are often cost – prohibitive for the developing world. Hence, ultrasound biometry is still very much in practice in large parts of the world. Using ultrasound-derived axial lengths in advanced formulae designed for optical biometry could lead to errors in IOL power calculation, even if optimized A-constants are used.
Given this disparity, there is a need to develop methods to integrate ultrasound measurements with advanced formulae such as the Barrett’s universal II, so that the accuracy of IOL power calculations improves universally and is not dependent on expensive technology, and affording patients can be offered premium IOLs with confidence. To solve this conundrum, we developed the Ladi’s adjustment to the ultrasound-derived axial length measurements and used this modified value with the Barrett’s universal II formulae. In this randomized study, we present comparative data showing the accuracy of this modified approach to IOL power calculation vs. optical biometry.
This was a prospective randomized study and was approved by the institutional ethics committee. The study followed the tenets of the declaration of Helsinki and an informed consent was obtained from all patients before enrollment.
All patients >40 years old, scheduled to undergo routine phacoemulsification with premium intraocular lens (IOL) implantation (by Johnson & Johnson Vision Care, Inc, USA) including monofocal (Tecnis), Eyhance IOLs, extended depth of focus (Symphony), multifocal (Tecnis multifical or Synergy) and toric IOLs (Tecnis toric, Symphony toric) from April 2021 to February 2022, were offered the opportunity to participate in the study and consenting patients willing to come for follow up till 6 months after surgery were enrolled. Only one eye per patient was enrolled. Eyes with coexistent pathologies likely to interfere with postoperative visual acuity such as corneal scarring, glaucoma and vitreoretinal diseases, with potential acuity of worse than 6/24 or having traumatic or complicated cataracts were excluded from the study.
Patients were randomized into 2 treatment groups for ocular biometry: the ultrasound method (US) or the IOL master 500 (IM500). Randomization codes were the random number assignment protocol available with STATA statistical analysis software. The codes were then placed in serially numbered sealed envelopes for allocation. The study coordinator revealed the type of biometry to be performed on the day the patient came back for IOL power calculations. The operating surgeon, optometrists and counsellors were masked to the randomization throughout the study, including at all postoperative visits.
The preoperative evaluation was performed by a trained optometrist. All patients underwent a comprehensive dilated eye examination including measurement of the best corrected visual acuity (BCVA), intraocular pressure (IOP) using non-contact tonometry, slit lamp evaluation to grade the density of cataract as per the lens opacification classification system III and a dilated fundus evaluation. The eyes randomized to the US group underwent keratometry and anterior chamber depth (ACD) assessment using a Sheimpflug based device (Costruzione Strumenti Oftalmici, Florence, Italy) where the central 3mm keratometry values were used after verifying the acceptable acquisition quality. The axial length was measured using the immersion ultrasound method (Echorule Pro, BioMedix Optotechnik & Devices Pvt. Ltd., Bangalore, India) in auto mode where the machine gives a reading only once all ideal immersion spikes and parameters have been obtained. Any reading in which the eye was not well aligned with the probe was shown in red, and that reading was deleted and thus excluded from the calculation. Dense cataract mode was used only in very dense and mature cataract cases where it was difficult to freeze spike patterns in ‘cataract’ mode. The gain automatically increased in the dense cataract mode to get optimal reading.
The axial length obtained was modified using the Ladi adjustment where 200 microns was added to the axial length value obtained from the ultrasound method. This value was chosen since partial low-coherence interferometry (PCI) based on the time-domain OCT used in the IM500 machine considers the distance between the internal limiting membrane (ILM) and Bruch’s membrane to be 200 microns [13]. Eyes randomized to the IM500 group underwent biometry including keratometry, ACD and axial length using the IOL master 500 (Carl Zeiss Meditech, Germany). All values obtained from the biometry was fed into the online Barrett’s universal II formula for IOL power calculation with the optimized optical A-constant provided by the manufacturer for the type of IOL being considered. The target refraction was kept at either zero or -0.5D when the Symphony IOL was being placed with the other eye being pseudophakic with a Symphony in situ. Surgery and postoperative evaluation: All cataract surgeries were performed by one surgeon (JL) using a temporal clear corneal incision using standard surgical techniques appropriate for the grade of cataract. The IOL placed was based on the patient’s choice and affordability. Postoperatively, patients were prescribed topical steroid eye drops for 1 month in a tapering fashion and topical antibiotics for 1 week. Patients underwent assessment of their BCVA and manifest refraction at postoperative months 1, 3 and 6. The spherical equivalent was calculated at the 6th month time point using the sphere + ½ cylinder values. The residual refractive error (RRE) was calculated as spherical equivalent – target refraction.
Our previous experience showed that 85% patients in the IM500 group would achieve their target refraction. We assumed that a 20% difference between groups achieving target refraction would be clinically meaningful, such that 65% in the US group with Ladi modification would achieve target refraction. Given this effect size, 1:1 randomization, an alpha error of 0.05 and an 80% power of the study, we required 83 eyes per group. Assuming a 25% loss to follow up, we enrolled 105 eyes in each group.
All continuous variables were expressed as mean with standard deviation or median with interquartile range and group differences between these were assessed using the student t-test for normally distributed data or the Mann Whitney U test for non-parametric distributions. Normality of distribution was assessed using the Wilcoxon’s ranksum test. The BCVA was converted to the logarithm of the minimum angle of resolution (logMAR) for analysis. Similarly, categorical variables were presented as proportions (n, %) and group differences were analyzed using the chi square or the Fischer’s exact test. A univariate and multivariable linear regression analysis was used to find factors influencing the RRE and all outcomes were presented as beta coefficients with 95% confidence intervals. All data were collected in Microsoft Excel and analyzed using STATA 12.1 I/c (Stata Corp, Fort Worth, Texas, USA). All p-values <0.05 were considered statistically significant. All analysis was carried out per protocol.
We included enrolled 210 eyes of 210 patients in the study of which 200 (n=100 each in the IM500 and US groups) came for follow up at 6 months and were used for analysis. The mean age of participants was 62.9 + 9.5 years and 103 (52%) were men. The two groups were comparable at baseline (table 1). The grade of cataract was NS2-3 in majority cases (n=152, 75%) with no differences between groups. The final IOL power used was 21.5 + 2.9D using the Barrett’s universal II formula. There were no differences in the groups in terms of monofocal, multifocal or toric IOLs used between the 2 groups (table 1). None of the eyes experienced any intraoperative complications such as posterior capsular rupture and had well-centered IOLs placed in the bag.
|
Variable |
US with Ladi adjustment |
IOLMaster 500 |
P-value |
|
Age |
61.9 + 9.6 |
63.8 + 9.2 |
0.22 |
|
Gender (% men) |
50 (50%) |
53 (53%) |
0.67 |
|
Preop Visual acuity (logMAR) |
0.47 + 0.53 |
0.42 + 0.49 |
0.46 |
|
Cataract grade: NS1 |
9 (9%) |
15 (15%) |
0.32 |
|
NS2 |
52 (52%) |
44 (44%) |
|
|
NS3 |
25 (25%) |
31 (31%) |
|
|
>NS3 |
14 (14%) |
10 (10%) |
|
|
PSC |
20 (20%) |
21 (21%) |
0.86 |
|
Preop IOP (mmHg) |
16.7 + 3.8 |
16.1 + 2.6 |
0.66 |
|
Mean K (Diopters) |
43.8 + 1.7 |
43.5 + 4.3 |
0.73 |
|
Axial length (mm) |
23.3 + 1.2 |
23.5 + 1.0 |
0.23 |
|
AC depth (mm) |
3.16 + 0.46 |
3.13 + 0.40 |
0.94 |
|
IOL power (Barrett)** |
21.24 + 3.4 |
21.05 + 2.6 |
0.47 |
|
IOL Type planned: |
|
|
|
|
Tecnis monofocal |
35 (35%) |
30 (30%) |
0.21 |
|
Eyhance (EDOF) |
1 (1%) |
9 (9%) |
|
|
Tecnis Toric (monofocal) |
17 (17%) |
20 (20%) |
|
|
Tecnis Multifocal |
17 (17%) |
14 (14%) |
|
|
Tecnis Toric (multifocal) |
1 (1%) |
3 (3%) |
|
|
Symphony |
13 (13%) |
14 (14%) |
|
|
Symphony Toric |
7 (7%) |
4 (4%) |
|
|
Synergy |
9 (9%) |
6 (6%) |
** Power Nearest to Zero, US: Ultrasound, Log MAR: Logarithm of Minimum Angle of Resolution, NS: Nuclear Sclerosis. PSC: Posterior Subcapsular Cataract, IOP: Intraocular Pressure, AC: Anterior Chamber, IOL: Intraocular Lens
A target refraction of zero was planned for 99 eyes in the US group and 94 eyes in the IM500 group. The exact target refraction was achieved in 73% eyes in both the US and IM500 groups (table 2), however 94% in the US group and 92% in the IM500 group achieved postop refraction within +0.5D of target (p=0.76). The RRE was comparable in the two groups (-0.09+0.27D in US vs. -0.08+0.23D in IM500, p=0.90) at 6-months. The RRE was slightly higher in eyes with AXL>25mm (n=18 eyes, -0.21+0.33D in US vs. -0.07+0.17D in IM500), though this was not statistically significant (p=0.27) (figure 1). Linear regression analysis showed that the preop BCVA (β= 0.06D, 95%CI= -0.5 to 0.13D, p=0.07) was the only factor marginally influencing RRE, with lower visual acuity associated with higher RRE. After adjusting for preop BCVA and axial length, the difference in RRE between the two groups was 0.02D (95% CI= -0.05 to 0.09, p=0.64).
|
Variable |
US with Ladi adjustment |
IOLMaster 500 |
P-value |
|
Targeted refraction |
-0.05 + 0.05 |
-0.03 + 0.12 |
0.07 |
|
Target refraction Zero |
99 (99%) |
94 (94%) |
0.06 |
|
Spherical equivalent (6m) |
-0.10 + 0.27 |
-0.11 + 0.25 |
0.61 |
|
Residual refractive error** |
-0.09 + 0.27 |
-0.08 + 0.23 |
0.90 |
|
1 month |
|||
|
BCVA |
0.10 + 0.22 |
0.07 + 0.14 |
0.32 |
|
Sphere |
-0.06 + 0.31 |
0.025 + 0.36 |
0.09 |
|
Cylinder |
-0.61 + 0.50 |
-0.70 + 0.50 |
0.85 |
|
3 months |
|||
|
BCVA |
0.08 + 0.16 |
0.06 + 0.12 |
0.53 |
|
Sphere |
-0.05 + 0.33 |
0.03 + 0.37 |
0.19 |
|
Cylinder |
-0.50 + 0.47 |
-0.61 + 0.50 |
0.87 |
|
6 months |
|||
|
BCVA |
0.07 + 0.15 |
0.09 + 0.28 |
0.95 |
|
Sphere |
-0.045 + 0.32 |
0.015 + 0.28 |
0.17 |
|
Cylinder |
-0.57 + 0.51 |
-0.65 + 0.47 |
0.88 |
|
% attaining target refraction |
73 (73%) |
73 (73%) |
0.99 |
** Residual refractive error= Spherical equivalent (6m) - Targeted refraction
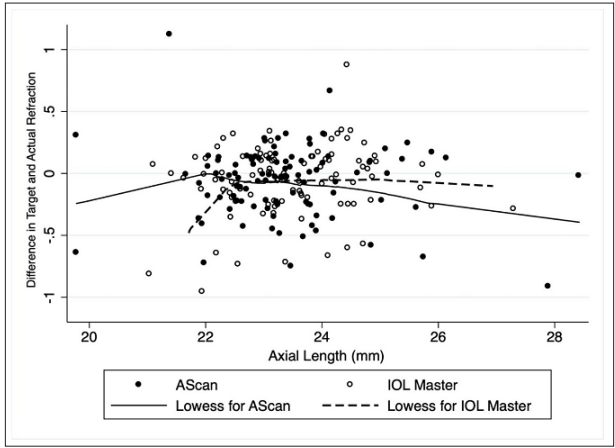
Figure 1: A Scatter plot with locally weighted curve showing the relationship between the axial length and the difference in target and actual refraction
In this randomized study, we found that the US biometry with the Ladi adjustment of axial lengths is as accurate as the IM500 with the same RRE, suggesting that it and can be reliably used with the Barret’s universal II formula for IOL power calculations. Caution should be maintained at extremes of axial length, though this requires further study. Preoperative visual acuity may be the only factor influencing RRE with lower vision leading to higher chances of errors. These findings imply that using the Ladi adjustment, the US-based IOL power calculations can be confidently used to offer premium IOLs to patients including multifocal, EDOF and toric IOLs, without the need for optical biometry which can be cost-prohibitive. Additionally, in patients with very dense or mature cataracts, where optical biometry cannot be relied upon, the ultrasound method with the Ladi adjustment can still be used to accurately to determine the IOL power.
The most accurate IOL power calculating formulae such as the Barrett’s universal II, Kane, Olsen and the Hill-RBF are all dependent on axial length measurements using optical biometers based on low-coherence PCI or swept-source. Hence these are quite difficult to use when there is no access to optical biometers. Previous studies have shown that optical biometry is more accurate than immersion ultrasound in calculating axial length, and that values derived from both these machines may not be used interchangeably [11,12]. Our method using the Ladi adjustment, where the ultrasound-derived axial length is enhanced by 200 microns, appears to overcome this problem, and provide refractive outcomes comparable to optical biometry using the low-coherence PCI based biometer. The 200-micron enhancement was based on the fact that the ultrasound measures axial length up to the ILM whereas the optical biometer measures the axial length up to the Bruch’s membrane. Theoretically, the difference between the ILM and Bruch’s membrane i.e., 200 microns should compensate for the difference in the axial length measured by the US and the IM500. We see this theory translating into practice where the 200-micron addition to the US-derived axial length gave very low RRE comparable to the IM500 derived axial lengths.
In addition to independence from optical biometers making IOL power calculation significantly cheaper, the Ladi adjustment helps offer premium IOLs to patients with dense nuclear cataracts, mature cataracts, dense posterior subcapsular and posterior polar cataracts, where the media opacity severely limits the ability of the optical biometers to accurately determine the axial length. This is important especially when considering multifocal and toric IOLs to such patients. Combining the Ladi-adjusted axial length with keratometry values derived from a Sheimpflug based corneal topographer improves the IOL power predictability even further. Corneal topographers are readily available in most clinics, even in resource poor settings, due to the popularity of corneal refractive surgeries as well as for detecting keratoconus and other ectasias early. This combination improves the surgeon’s confidence when offering premium IOLs, as seen from our study where all premium IOLs have been offered even to the ultrasound group.
The main drawbacks of this study are the lack of comparison of the Ladi adjustment of US-derived axial length with more sophisticated methods using swept-source optical biometry. The major advantage is the utilization of a randomized study design with relatively good numbers and adequate follow up time to detect refractive outcomes. To the best of our knowledge, there are no studies looking at making US-derived axial lengths compatible with newer IOL calculating formulae. These results should be limited to the Barrett’s formulae and cannot be extrapolated to other formulae without more study. In conclusion, the Ladi adjustment of adding 200 microns to the US-derived axial length and combining this modified value with Sheimpflug based keratometry in the Barrett’s universal II formula provided comparable refractive outcomes with minimum errors compared to the IM500 based values. Further studies are required with larger sample size as well as with other evolving IOL calculation formulae to determine more widespread adoption of this simple adjustment method.
