Author(s): Ramachandran Muthiah
Costello syndrome is a rare RASopathy resulting from germline mutations of the protooncogene HRAS. Many of these mutations affect SHP2, SOS1, RAS, RAF and MEK proteins it was discovered by Dr. Jack Costello, a New Zealand paediatrician in 1977. Dr. White says. Costello syndrome is now known to be one of a group of related disorders,, caused by abnormal functioning of the Ras?mitogen?activated protein kinase (RAS/MapK) pathway. Ras/MAPK pathway is an essential signaling pathway that controls cell proliferation, differentiation, survival and its dysregulation causes clinically overlapping genetic disorders, called as ‘Rasopathies’.In this pathway, Ras, a GTPase, transmits extracellular signaling from receptor tyrosine kinases to two serine/threonine kinases (Raf and MEK) and, finally, to the activation of MAPKs. Costello syndrome is a severe developmental disorder characterised by postnatal growth retardation with delayed skeletal maturation, psychomotor retardation, cutis laxa, and acanthosis nigricans. Excessive mucopolysaccharides, which accumulate in cultured fibroblasts of patients with Costello syndrome (CS). Intracellular accumulation of chondroitin non-sulphate, as a cause of functional deficiency of the 67 kDa elastin binding protein, has been described in fibroblasts of patients with Costello syndrome.This gives support to the previous hypothesis of a defect in lysosomal degradation.
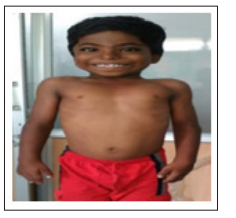
Individuals with CS have a characteristic craniofacial phenotype. The majority (>50%) of subjects presented with relative macrocephaly (93%), high forehead (85%), bitemporal narrowing (98%). hyperteloric (56%) and telencanthic (83%) appearance. Most CS individuals had a short nose (73%) with a depressed nasal bridge (73%) and wide nasal tip (90%). Individuals with CS also had full cheeks (71%), largemouth (76%), and thick vermillion to upper lips (95%), often with a tented upper lip (76%). Commonly in CS, ears were low-set (93%) and posteriorly rotated (73%) with upturned lobes (66%). Signs of cutaneous paraneoplastic syndrome such as papilloma, pachydermatoglyphia (tripe palms- based on the similarity of rugose appearance of cow’s stomach, exaggerated ridging pattern on palms and fingertips), palmoplantar keratoderma and acanthosis nigricans. A dental malocclusion in which the maxillary posterior teeth are positioned lingually (i.e., toward the tongue) relative to mandibular teeth (normally, maxillary teeth are placed bucally [i.e., toward the cheek]), was significantly more common in the CS cohort (35%). Abnormal elastin distribution on tortuous dilated arteries and veins in pulmonary vasculature, causing nonuniform, well thickened, obliterative lesions at arterial branch points leading to early pulmonary hypertensive vascular disease. Structural malformations of the heart present at birth such as valvular pulmonic stenosis and abnormal thickening of the muscular walls of the ventricles (ventriculomegaly). Most worrying aspect of the CS phenotype. Frequency of malignancy in mutation positive cases is 11%. In the Japanese and North American series, the commonest mutation was the G12S missense substitution. In G12Ser mutation, rhabomyosarcoma was observed in (7%), with a benign bladder tumour in a single case and the, more severe neoplastic phenotypes are p.Gly 12 Asp and p.Gly 12 Cys. The features of increased index of suspicion of CS in newvorn are fetal atrial tachycardia, increased birth weight and head circumferencw, neonatal hypoglycemia, severe feeding difficulties and urinalysis for hematuria ( embryonal rhabdomyosarcoma) and loose, redundant skin on the hands and feet seen in newborns (key role in clinical suspicion of CS). Ras pathway agents, such as farnesyl transferase inhibitors (tipifarnib and lonafarnib) that prevent posttranslational modification of Ras, are being evaluated and may be of therapeutic use for syndromes in this pathway, especially CS. MEK 162 (Binimetinib), orally available inhibitor of mitogen-activated protein kinase kinase 1 and 2 (MEK1/2), directly target the RAF-MEK-ERK 1/2 cascade and it is the best tool against cardiac hypertrophy. The most established mTOR inhibitors are so-called rapalogs (rapamycin and its analogs) reverse cardiac defects. Simvastatin- interact with Ras isoprenylation, decrease Ras activity and low dose statin with selective inhibition of pathological ERK 1/2 signaling is an exciting possibility in the treatment of CS patients. MEK inhibitors rescue the enamel mineralization defect in Costello syndrome. PI3K inhibition only corrects the progenitor cell proliferation phenotype. RAF-1 inhibition by C-type Natriuretic Peptide (CNP) improved bone growth in preclinical animal models.
The Splicing Efficiency of Activating HRAS Mutations Can Determine Costello Syndrome Phenotype and Frequency in Cancer. This unravels a potential for development of new anticancer therapies based on SSO-mediated HRAS exon 2 skipping. Gene correction of these germ line mutations to restore normal protein functions is anticipated as a new therapeutic option. This can be achieved through disruption of gain-of-function pathogenic mutation, restoration of loss-of-function mutation, addition of a transgene essential for cell function and single nucleotide changes. Development of genome editing tools comes in two waves. The first wave focuses on improving targeting specificity and editing efficiency of nucleases. The second wave of gene editing draws on innovative engineering of fusion proteins combining deactivated nucleases and other enzymes that are able to create limitless functional molecular tools. Technology CRISPR (clustered regularly interspaced short palindromic repeat) has been nothing less than miraculous, at the cellular level to fix the genetic diseases by editing the double helix of the DNA. The tool acts as a pair of DNA scissors to modify the genes by replacing the defected ones. This technology has had a revolutionary impact on the life sciences, is contributing to new cancer therapies and may make the dream of curing inherited diseases come true.
Oxidative stress- play a role in cancer development and free radicals- determine non-neoplastic clinical features such as elastin anomalies, alteration of skin and appendages, developmental retardation and cardiac defects. PAR therapy (potassium ascorbate with ribose) a reduction in oxidative stress biomarkers in parallel with improvement of clinical features. It combines the antioxidant action of vitamin C with the stabilizing intracellular effects of potassiu and causes improvement of skin and appendage lesions, better evolution of psychomotor development, no Progression of heart hypertrophy, nor tumor development. It is low cost, no sideeffects, orally administered and useful for all genetic syndromes with cancer risk [1-5].
References
