Author(s): Joshua Parker Elijah, Anorue Eleazar Chukwuemeka*, Obidoa Onyechi, Nwodo O Fred C, Esimone Charles Okechukwu and Nworu C S
Cancer is a disease involving abnormal cell growth with the potential to invade or spread to other part of the body. Presently cancer has been declared as a global burden by the World Health Organization and the second-leading cause of death in the world. An estimated 26,000 people die each year of cancer and in the year 2018 alone, an estimated 9.5 million people died of cancer worldwide. Particularly in Africa, the burden of cancer and cancerrelated diseases is on the increase, due to inefficient prevention strategies and poor prognosis following late diagnosis [1-3].
Among all the forms of cancer, pancreatic cancer, the cancer of the pancreas is the fourth leading cause of cancer death. It is the tenth most common cancer in men and the eighth most common in women. The disease remains elusive to effective screening approaches and 80% of cases presents an unresectable or incurable stage. The symptoms do not appear until the later stages [4-7].
Some of these symptoms are abdominal or back pain, jaundice, low appetite, weight loss, swelling of the gall bladder or liver, blood clots, deep vein thrombosis, pulmonary embolism, diabetes, pale gray or fatty stool, nausea and vomiting, fever, and chills, fatigue, diarrhea or constipation and indigestion [8]. Two major types of pancreatic cancer are the exocrine tumors and the neuroendocrine tumors [9-10]. About 93% of all pancreatic tumors are exocrine and the most common kind of pancreatic cancer is called adenocarcinoma [11]. Pancreatic adenocarcinoma is what people usually mean when they say they have pancreatic cancer [12]. The most common type begins in the ducts of the pancreas and is called ductal adenocarcinoma; it accounts for 90% of pancreatic cancer [5].
Treatment for cancer is mainly by surgery, chemotherapy or radiation and usually expensive [10]. However, recent researches have shown that medicinal plants have proven efficacious in the cure/treatment of different diseases [13]. Recently, the value of medicinal plants as a promising source of active drugs has gained more interest due to availability, cost-efficiency, safety, and better bioactivity with less or no side effects [14]. Bitter Leaf (Vernonia amygdalina), tomato (Solanum lycopersicum) and coconut (Cocos nucifera) have been reported to possess many therapeutic effects [13]. For instance, bitter leaf has shown to possess anti-bacteria, antimalaria, anti-parasitic, hypoglycaemic and hypolipidaemic properties among others [15]. Tomato is used to treat diabetes,high blood pressure, heart disease, osteoarthritis and many other conditions while coconut possesses antimalaria, anti-inflammatory, antioxidative, antibacterial and antidiabetic properties [16-17]. Recent reviews have suggested that these plants also possess cytotoxicity to some human tumor cells [17-19]. However, it is uncertain whether these plants possess any cytotoxic effect on pancreatic cancer. Therefore, this work was designed to determine, through cell lines, the cytotoxic responses of pancreatic cancer cell (PANC-1) to the reconstituted lyophilized extracts of coconut milk, bitter leaf and tomato.
The samples (Vernonia amygdalina, Solanum lycopersicum and Cocos nucifera) were collected in Nsukka, Enugu State, Nigeria. The plant samples were identified and authenticated by A. Ozioko of the International Centre for Ethnomedicine and Drug Development (BDCP), Nsukka, Nigeria, where the voucher specimens were deposited and some in the Department of Botany, University of Nigeria, Nsukka.
The reconstituted lyophilized plant extracts of coconut milk, bitter leaf and tomato was prepared according to the method of [20]. Briefly after washing and drying the leaves, the leaves were ground using a mechanical grinder. Thereafter was a known weight of the pulverized leaves (3000g) was macerated in 10 L absolute ethanol each using a maceration flask. The mixture was left for 72 hours with occasional stirring, after which it was filtered into a flat-bottomed flask using a muslin cloth. Further filtration was achieved with Whatman No 1 filter paper so as to remove fine residues. The filtrate was concentrated in-vacuum to obtain the crude ethanolic extract. Thereafter, lyophilization was observed to the best process to obtain the dry extract of plant extracts.
The cancer cell line used was immortalized pancreatic (PANC-1) cancer cell line which was kindly provided by Dr. Oliver Wildner of the Department of Molecular and Medical Virology, Ruhr University, Bochum, Germany
The cell lines were propagated in D-10, consisting of Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, 2 mM L-glutamine and supplemented with 10% heat-inactivated foetal bovine serum (FBS), 100 U ml-1 penicillin and 100 μg ml-1 streptomycin. Tissue culture medium and supplements were purchased from Invitrogen (Karlsruhe, Germany). The cell cultures were maintained in a humidified 5% CO 2 atmosphere at 37°C.
Lyophilizer (Model Modulyo 4K, Edward, England), Water Bath (Gallenkamp, England), Chemical Balance (Gallenkamp, England), Conical Flasks (Pyrex, England), Hotbox (Gallenkamp, England), Centrifuge (3,500 rpm, PIC, England), Digital Photo Calorimeter (EI 312 Model, Japan), Adjustable Micropipette (Perfect, U.S.A.), Refrigerator (Kelvinator, Germany), pH Meter (Pye, Unicam 293, England), multi-well microtiter plate reader (Tecan, Austria).
The cytotoxicity assay was performed on the extracts using the MTT assay method as previously described on human immortalised pancreatic cancer cell line (PANC-1) [21]. In the MTT assay, cells were seeded onto a 96-well plate at a concentration of 104 cells/ well and a volume of 100 μL per well. Various concentrations of the test compounds ranging from 125 to 2000 μg/ml in culture solubilised in DMSO were applied to culture wells in triplicate. Culture medium also containing DMSO was used as the “no-drug” control. After incubation at 37°C under 5% CO 2 for 2 days, a solution of MTT (3 mg/ml, 50μl per well) was added to each well and further incubated at 37°C + 5% CO2 for 1 h to allow formazan production. After this time, the medium was removed and 150 μl of DMSO was used to dissolve the resulting blue formazan crystals in living cells. The optical density was determined at 550 nm using a multi-well microtiter plate reader (Tecan, Austria). Each single value of the triplicates was expressed as percent of the mean of triplicates of the “no-drug” control cultures and the mean and standard deviation of the percent values would be calculated for each triplicate. The concentration of 50% cellular toxicity (TC50) of the test extracts was calculated by non-linear regression analysis.
Coconut milk, bitter leaf and tomato extracts showed a potent concentration and dose-response effect on the growth inhibition of a pancreatic cancer cell lines (PANC-1) as shown in Figures 1, 2 and 3. The extract of bitter leaf exhibited the most potent effect on PANC-1 cancer cell line with a regression concentration of 237.10 ± 130.30 μg/ml and was closely followed by tomato extract (520.10 ± 172.3 μg/ml) which suppressed the cell viability of PANC-1 to less than 50%. On the other hand, coconut extract reduced the cell viability to less than 50% but with higher regression concentration value of 1917.0 ± 124.9 μg/ml; hence the least potent extract of all the extracts under study.
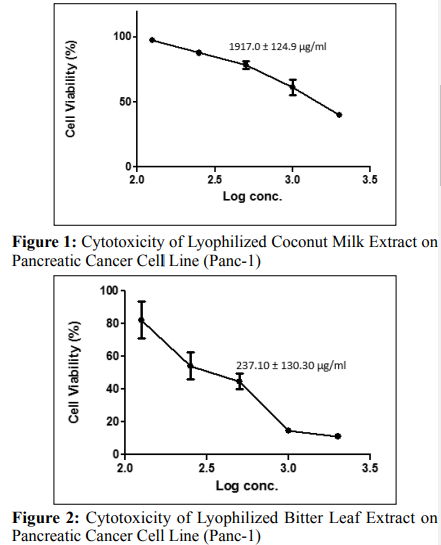
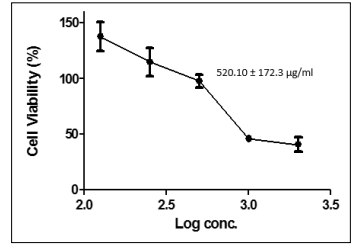
Figure 3: Cytotoxicity of Lyophilized Tomatoes Extract on Pancreatic Cancer Cell Line (Panc-1)
The quantification of cellular growth, including proliferation and viability, has become an essential tool in any laboratory working on cell-based studies [6]. Such techniques enable not only the optimization of cell culture conditions, but also the determination of growth factor and cytokine activity [7]. Even more importantly, the efficacy of therapeutic agents in drug (including plant extracts) screening, the cytostatic potential of anticancer compounds in toxicology testing, and cell-mediated toxicity can be assessed when quantifying cell growth [6]. The first step in any experiment is the decision whether to test for cell proliferation or viability parameters, depending on the objective of a study [22]. These parameters are measured by assaying for “vital functions” that are characteristic for healthy or growing cells [5]. But the scope of this cytotoxicity study hinges on cell viability expressed in percentage [21].
Cell viability measurements assess healthy cells in a sample [5]. This can be accomplished either by directly counting the number of healthy cells or by measuring an indicator for healthy cells in cell populations (e.g. in a microplate assay) [6]. Most viability assays are based on one of two characteristic parameters: metabolic activity or cell membrane integrity of healthy cells [18]. Usually, the metabolic activity is measured in cell populations via incubation with a tetrazolium salt (e.g., MTT, XTT, WST-1) that is cleaved into a coloured formazan product by metabolically active cells [19]. Also, these cell line studies anchor on the assessment of cell viability through the assay of metabolic activity using MTT as a source of tetrazolium salt [17].
Potent concentration and dose-dependent effects were observed (Figures 1, 2 and 3) when the lyophilized samples (coconut milk, tomatoes and bitter leaf) were applied to immortalized human pancreatic cancer cell line (PANC-1) at the regression concentration values of 883.90 ± 77.89 μg/ml, 281.5 ± 29.52 μg/ ml and 1025.0 ± 44.89 μg/ml for coconut milk, bitter leaf and tomatoes respectively. The cytotoxicity assay on the PANC-1 cell line as shown in Figures 1, 2 and 3 shows that cell viability decreased with corresponding increase in log concentration of the applied samples or extracts with bitter leaf showing the most potent and appreciable effect on the PANC-1 cancer cell. However, tomato extract gave the least potent effect compared with bitter leaf and coconut extracts. Significantly, the regression concentration and dose values (281.5 ± 29.52 μg/ml and 237.10 ± 130.30 μg/ ml) of bitter leaf were found to be the least in the cancer cell line analysis (PANC-1); despite that, it still showed a more potent effect by decreasing the percentage of cell viability with increasing log concentrations of the extract.
An increase in cell viability indicates cell growth, while a decrease in viability can be interpreted as the result of either toxic effects of compounds/agents or suboptimal culture conditions [18]. The above promising results of the three lyophilized extracts (coconut milk, bitter leaf and tomatoes) on the assayed cancer cell lines may be attributed to variety of antioxidant presents in the plant extracts [14]. Phytochemical screening of bitter leaf showed the presence of phenolic content in the leaves which indicates its antioxidant properties [13]. Phytochemical profiling of coconut milk also showed coconut is rich in antioxidants such as catechin, salicylic acid, gallic acid, caffeic acid, salicylic acid and p-coumaric acids [17]. While the phytochemical screening of tomatoes showed the presence of antioxidants such as lycopene, vitamin C and vitamin A [16]. The mechanism by which these antioxidants exert their anticancer effects could be through several possible mechanism such as removal of carcinogenic agents, modulation of cancer cell signalling and antioxidant enzymatic activities and induction of apoptosis as well as of cell cycle arrest. For instance, a study on the anticancer effect of bitter leaf attributed its anticancer effect to induction of cell cycle arrest and apoptosis through the suppression of the expression of cyclin D1 and cyclin E via a p53 independent pathway [14]. Another study on the anticancer effect of tomatoes attributed its anticancer properties to decrease of free radical damage to the DNA that can lead to cancer [16]. While another study on the anticancer effect of coconut attributed its anticancer effect to modulation of gene function, carcinogenmetabolizing enzymes, apoptosis and immune function [17]. The highest potent effect shown by bitter leaf extract could be due to higher presence of antioxidants compared to the extract of coconut milk and tomatoes [14].
For human model of the research conducted, the results from this study using the immortalized pancreas cancer cell line (PANC1) suggest that a low-fat and high-fibre diet such as coconut and other antioxidant-rich diet sources such as bitter leaf and tomatoes which possess lycopene might be effective for halting or slowing the progression of early-stage cancers but would be of lesser value for end-stage cancers [15]. The food/diet sources under study may possess some pharmacological potentials to alter serum factors that reduce growth and increased apoptosis in serum-stimulated, cancer-induced cell (PANC-1) [23-25].
In conclusion, the results obtained in this study showed using immortalized pancrease cancer cell line (PANC-1) suggest that a low-fat and high-fibre diet such as coconut and other antioxidantrich diet sources such as bitter leaf and tomato which possess lycopene (a potent antioxidant) might be effective for halting or slowing the progression of early-stage pancreatic cancer but may be of lesser value for end-stage cancers. The food/diet sources under study may possess some pharmacological and anticarcinogenic potentials to alter serum factors that reduce growth and increased apoptosis in serum-stimulated, cancer- induced cells such as immortalized human pancreatic cancer cells (PANC-1).
Well acknowledged is Dr. Oliver Wildner of the Department of Molecular and Medical Virology, Ruhr University, Bochum, Germany who kindly provided the immortalized human pancreatic cancer cell line (PANC-1). Then, of course, there were the combined efforts towards the correction/editing of the work by Prof. E. O. Esimone, Dean, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Anambra State of Nigeria, and Dr. C. S. Nworu and staff of the Department of Molecular andMedical Virology, Ruhr University, Bochum, Germany, who are gratefully acknowledged for the unquantifiable and immeasurable assistance on the cell line work. Laboratory attendants of the Analytical Laboratory of the International Institute for Tropical Agriculture, Ibadan are also acknowledged for some of the painstaking preliminary laboratory analyses. Not forgetting my Ph.D supervisors - Prof O. Obidoa and Prof. O. F. C. Nwodo.
All authors made significant contributions to the conceptualization and design of the study, read and approved the final manuscript.
Declaration
Consent for publication
All authors have gone through the manuscript and given their
consent for publication
Not applicable
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
No funding was received for this work
