Author(s): Mesheal AL-Abdulrahman, Yasir Abdullah bin Khaliffah, Muteap bahni alhaily, Meshael Oudah Dawi AlOtaibi, Amal faisal Almutairi, Haneen Madailah Aldirbas, Ahoud Faisal Almutairi, shrouq Faisal Alhazmi, Norah Oudah Alotaibi, Halia Faya Asiri and Abdul-Wali Ajlouni*
Counterfeit medicines are one of the major challenges facing pharmaceutical supply chains and patient safety and are a serious problem in countries around the world. These drugs represent a major threat to the patient’s life, to the real drug manufacturers, and to the country’s image. The problem of substandard/ counterfeit/counterfeit/counterfeit medical products is more serious in countries with weak health regulatory systems and fragile health infrastructure. These characteristics increase the risk of manufacturing and/or distributing medicinal products that do not comply with national and regional health regulations, putting patients at risk. High and variable prices, insufficient access to affordable medications, and drug shortages are incentives for actions, activities, and behaviors that lead to SSFFC medical products. These problems must be addressed from a public health perspective. In this paper, the authors will provide a direct and simple comparison between the five most popular detection techniques used to analyze and characterize various genuine and generic pharmaceutical brands, as an approach to analyzing counterfeit pharmaceutical products, as many genuine and generic products are classified as counterfeit and vice versa. The combination of analytical results provides a comprehensive characterization of counterfeit medical products. This information is essential for patient safety and to assist authorities in their mission to combat counterfeit products. The findings could help police, regulatory, and health bodies reduce the burden of combating counterfeit medicines and the consequences that occur if they are not detected and used.
The global crisis of the Covid-19 pandemic has led to increase the trade of counterfeit medicines, due to the shortage in the supply of some medicines to provide increasingly counterfeit alternatives. The most counterfeit drugs-related to Covid-19, were including the anti-malarial, anti-viral chloroquine, vitamin C, antibiotics, pain relievers, herbal medicines, and medical supplies such as face masks, coronavirus test kits, ventilators, gloves, disinfectants, gels, and wipes. Cleaning, soap, where the sale of counterfeit vaccines has been reported in certain countries of the world [1-3]. A counterfeit drug is a pharmaceutical product that is produced and sold with the intent to deceptively represent its origin, authenticity, or effectiveness. It may contain inappropriate quantities of active ingredients, may be improperly processed within the body, may contain ingredients that are not on the label, and is often sold with inaccurate, incorrect, or fake packaging and labeling. Counterfeit drugs are one of the key challenges facing pharmaceutical supply chains and the safety of patients, and it is a serious problem in countries all over the world. These drugs represent a great threat to the patient’s life, the genuine pharmaceutical manufacturer, and the image of the country. According to the World Health Organization (WHO), 25% of medicines consumed in poor countries could be counterfeit or below standard, as the manufacturing costs are 40% cheaper here as compared to other countries. It is estimated that 40 percent of the pharma market, is under the grip of spurious and black-marketed drugs. Not only is the people’s health at stake but also there is a serious loss to the inspector of governments as they are deprived of huge amounts on account of sales tax and excise duty. Proper drug quality monitoring, enforcement of laws and legislation, an effective and efficient regulatory environment, and awareness and vigilance on the part of all stakeholders can help tackle this problem [4]. The pandemic has created a public health gap that has strained almost all health care resources to the breaking point. There was a shortage in the drug supply chain due to Covid-19 because demand exceeded the supply of drugs, including tranquilizers, analgesics, and antiviral products, and the closure of countries and borders of manufacturing sites led to the disruption of the supply chain for some drugs, active pharmaceutical ingredients, and raw materials [5-7]. The substandard/Spurious/Falsely-labeled/Falsified/ Counterfeit (SSFFC) medical products problem is more severe in countries with weak health regulatory systems and fragile health infrastructures. These characteristics increase the risk that medical products that are not in compliance with national and regional health regulations will be manufactured and/or distributed, putting patients at risk. High and variable prices, inadequate access to reasonable medicines, and drugs in shortage are incentives for actions, activities, and behaviors that result in SSFFC medical products. These problems must be tackled from the public health perspective [8]. Since counterfeit drugs are becoming increasingly more sophisticated, additional analytical techniques are necessary to detect these counterfeits. Qualitative and quantitative analysis of pharmaceutical active ingredients could be achieved by different chromatographic and spectroscopic methods. Various analytical techniques such as Liquid Chromatography-Mass Spectroscopy (LC-MS), Gas Chromatography-Mass Spectroscopy (GC-MS), Liquid Chromatography - Tim-Of-Flight Mass Spectrometer (LC- TOF), Liquid Chromatography- Photometric Diode Array (LC- PDA or DAD), Nuclear Magnetic Resonance (NMR) and Raman/ Infrared Spectroscopy (IR) are used to determine the counterfeit product. In this paper, a comparison between different analytical techniques will be done, where five analytical methods: UV-Vis, GC-MS/MS, LC-MS/MS, LC-PDA and UPLC-Q TOF, which are used as the most efficient technique to investigate and analyze genuine and generic drugs, as an approach to analyze falsified pharmaceutical products, since several genuine and generic are classified as counterfeits and vice versa. Combining the analytical results provides a comprehensive characterization of the falsified medicinal products. This information is essential for patient safety and to help authorities in their task of combatting falsified products. Subsequently, to examine whether there is a difference in the result between the five analytical methods, and how that will be used in detecting and limiting the spread of counterfeited drug. This will be followed by an examination of the proposition that this method can aid police and other regulatory agencies and health systems in reducing the burden of fighting counterfeit drugs, and the consequences that happen if they are not detected and used. Detecting and destroying this fake medication will prevent this criminal act and provide safer choices in consuming and dispensing this medicine. Studies of illicit drugs to obtain their chemical or physical profiles are intended either to prove the existence of a crime or to get accurate and precise information to the strategic and operational intelligence services. A profile can be defined as a series of specific characteristics selected to provide information about certain secret production for samples of synthetic drugs in tablet pharmaceutical dosage form. The purpose of this paper is to present a comparison between different analytical methods for their detection and the challenges faced in screening and identification of fake medication. This information could be very useful in the detection of all medications. In the following sections, detailed descriptions of the analysis of different counterfeited drugs using different analytical techniques are presented. The main results achieved and a broad discussion of drug security, including an action plane to achieve a secure drug in industry and handling.
The first definition of counterfeit medicine was proposed by (WHO) in 1992: “A counterfeit medicine is one which is deliberately and fraudulently mislabeled with respect to identity and/or source; and counterfeit products may include products with the correct ingredients or with the wrong ingredients, without active ingredients, with an insufficient active ingredient or with fake packaging”. In 2011, new terms were proposed by WHO were “Substandard, Spurious, Falsely Labelled, Falsified and Counterfeit” (SSFFC), considering the public health impact. Recently, a proposition was made by WHO to replace those terms by the expression “substandard and falsified medical products” (WHO). There is an actual global consensus to accept the expression “falsified product” which includes various misrepresentations of medicines in terms of their identity, composition and source, although the notion of counterfeit products is still widely used by the scientific network [9]. The classification of SSFFC medical products permits a more thorough and accurate comparison and analysis of these medications, separating substandard medical products from those that are deliberately/fraudulently making a misrepresentation and those that are unregistered/unlicensed in the country of marketing. Substandard: authorized medical products that fail to meet either their quality standards or their specifications, or both. Spurious or Falsely Labelled: the label or container bears the name of a company purporting to be the manufacturer of the drug, but this company is fictitious or does not exist; or, it purports to be the product of a manufacturer of whom it is not truly a product. Falsified: Medical products that deliberately/fraudulently misrepresent their identity, composition or source. Counterfeit: a counterfeit medicine is one which is deliberately and fraudulently mislabeled with respect to its identity and/or source (WHO, 2019a).
The main stages of production of pharmaceutical tablets may be listed as follows:
Actions, activities, and behaviors that result in SSFFC medical products are a local and global health problem related to the integrity of the manufacturing and supply chain that must be prevented, detected, and effectively responded to. Investigation for counterfeit medicines remains particularly limited, despite their strong global public health threats, with available data indicating an increasing global criminal trade that prevails the necessity to be addressed appropriately. Efforts for combating this illicit trade by a variety of public and private sector organizations, national administrations, and international groups, are tangled by weak governance and divergent interests. Even WHO efforts failed to incorporate the necessary international partners to combat this problem. This droves other international organizations such as UN Office of Drugs and Crime, UNODC, and Interpol to have an important role in combating counterfeit medicines (Mackey and Liang, 2013).
There are several ways to detect SSFFC medications, ranging from the visual examination of overt marks to full chemical analysis in the laboratory. In the field of chemical analysis, a wide range of technologies are also available, scanning portable devices and high-technological forensic laboratories. While laboratories can provide the most complete analysis, some portable devices have shown an accurate identification of SSFFC medications. The use of detection technologies should include active pharmaceutical ingredients and excipients as well as dosage from medical products.
Visual examination is the simplest detection methodology and is mostly related to the described overt marks. This examination usually acts on or involves the use of the sense organs observation of the product with the naked eye. It can also be performed with the aid of some device, e.g., a microscope or hand-held reader. Even in the absence of specific authentication marks, observation, measurement or analysis are the possible methods to identify SSFFC medications. For example, tablet weights and dimensions are tightly controlled and characteristic for any given formulation, making detection of SSFFC medications easy by their difference from the original product. This may also be true for the physical appearance and characteristics of the packaging components, particularly when these are tightly specified and controlled by the manufacturers. In view of the high standards to which licensed manufacturers operate, errors in drawings, texts, or graphics, may represent additional consistent signs. A higher grade of expertise, knowledge, and specified equipment may be essential to complete the examination and evaluation for the analysis of covert authentication tools.
Given the wide range of products and practices that come under the “counterfeit” umbrella, few methods are available for the identification and prevention of counterfeit medicine. Amongst the anti-counterfeiting technologies that have been developed are those based on stable isotope analysis in which the unique chemical properties of a substance provide forensic evidence of its manufacturing process or the raw material used. In addition, a collection of briefs but detailed methods to evaluate identity, effectiveness, or impurity profiles, may provide proper information by which suspect materials are valid. It is important to note that the identification and/or effectiveness of the active medical component in a suspect amount is not adequate to determine the genuineness of a suspect material. Forensic/chemical investigations comprise chemical, physical or forensic tests that may be conducted in the field by portable detection devices and technologies, or in a laboratory using a lab setting and equipment. These investigations may be utilized in the examination of the whole medication form and/or packaging material. There are several physical and chemical testing technologies that are designed to offer evidence that a material is SSFFC. Examples include spectroscopic and chromatographic tests, chemical-induced color-based testing, and hardness and dissolution tests. Chemical investigation is conducted using methods and technologies available to the competent governmental authorities or the manufacturer and that evaluate the composition of a suspicious product, and they are frequently the best conclusive method to confirm its originality. Increasingly, however, many instrument companies are looking for ways to innovate these technologies to be more cost-effective and user- friendly. At the same time, cheap, portable/hand-held instruments that is easily functioning is becoming more available. Such portable instruments can assist competent governmental authorities, law enforcement officers, and customs officials in scanning suspect materials at distant locations or ports of entry. Mostly, results made by a portable device in the field are considered primary, and approval is required through laboratory analysis. The most common fraudulent practice involves a medicine that contains the wrong or no active ingredient, or even the right active ingredient but at the wrong dose and the most serious one is to contain manipulated materials like heavy metals and other elements. There are however other more sophisticated practices such as the deliberate copying of existing patents for processes or formulations or the relabeling of stolen drugs or of their origin to avoid anti- dumping measure or to benefit from a “clear status” of a producing country (Jamin et al., 2013). Many methods and techniques have been used to detect and identify counterfeited drugs. Techniques like ultraviolet/visible (UV-Vis) spectrophotometry, fluorimetry, titrimetry, electro-analytical techniques, chromatographic methods, comprising thin-layer chromatography (TLC), gas Chromatography (GC), and high-performance liquid chromatography (HPLC)), capillary electrophoresis (CE), vibrational spectroscopies, Raman spectroscopy and hyper-spectral imaging are the main techniques that have been used for the quantitative analysis of pharmaceutical spurious compounds. While infrared (IR) spectroscopy can primarily detect non-genuine features on the packaging and identify some of the compounds present in the samples, only by spectra inspection, mass spectrometry allows the study of impurity profiles related to the Active Ingredient Pharmaceutical (API) and often the detection of known substitute API’s. In the following paragraph, a short preview will be introduced for the UV-VIS, GC-MS/MS, LC-PDA, LC-MS/MS and UPLC-Q-TOF analytical methods which is used for identification and determination of counterfeit medications.

Figure 1: UV-VIS Spectrophotometric in CFS labs
Spectroscopy methods is the branch of science dealing with the study of interaction between Electromagnetic radiation and matter. It is a most powerful tool available for the study of atomic and molecular structure/s and is used in the analysis of wide range of samples. Optical spectroscopy includes the region on electromagnetic spectrum between 100 Å and 400 μm. UV-VIS spectrophotometry is one of the most frequently employed techniques in pharmaceutical analysis, where several UV-Vis tests are widely developed for analyze counterfeit medication. As most prescription drugs possess chromophores groups, they will be determined directly within the ultraviolet region while not the necessity for a derivatization reaction. It involves measuring the amount of ultraviolet or visible radiation absorbed by a substance in solution. Instruments that measure the ratio, or function of ratio, of the intensity of two beams of light in the UV-Visible region are called Ultraviolet-Visible spectrophotometers. In qualitative analysis, organic compounds can be identified by use of a spectrophotometer, if any recorded data is available, and quantitative spectrophotometric analysis is used to ascertain the number of molecular species absorbing the radiation. The spectrophotometric technique is simple, rapid, moderately specific, and applicable to small quantities of compounds. The fundamental law that governs the quantitative spectrophotometric analysis is the Beer-Lambert law. The intensity of a beam of parallel monochromatic radiation decreases exponentially with the number of absorbing molecules. In other words, absorbance is proportional to concentration, this is known as Beer’s law. The intensity of a beam of parallel monochromatic radiation decreases exponentially as it passes through a medium of homogeneous thickness, this is known as Lambert’s law. A combination of these two laws yields the Beer-Lambert law. The intensity of a beam of parallel monochromatic radiation decreases exponentially with the number of absorbing molecules. In other words, absorbance is proportional to concentration, this is known as Beer’s law. The intensity of a beam of parallel monochromatic radiation decreases exponentially as it passes through a medium of homogeneous thickness, this is known as Lambert’s law. A combination of these two laws yields the Beer-Lambert law (indiastudychannel.com, 2017). Quantification of medicinal substances using a spectrophotometer may carried out by preparing a solution in a transparent solvent and measuring its absorbance at a suitable wavelength. The wavelength normally selected is the wavelength of maximum absorption (λmax), where a small error in setting the wavelength scale has little effect on measured absorbance. Ideally, concentration should be adjusted to give an absorbance of approximately 0.9, around which the accuracy and precision of the measurements are optimal.
A mass spectrometer has been developed to be used as a detector in gas chromatography during the mid of twentieth century, by Roland Gohlke and Fred McLafferty. These devices which were originally limited to laboratory settings, are sensitive, bulky, and fragile. The mass spectrometer is a device used for analytical purposes. It is a necessary tool in the fields of chemistry, pharmacy, food, forensics, and biochemistry. The first attempts at using this technique started in 1912 and from then on, there have been constant improvements. Nowadays we have a technique, which is reliable, sensitive, and fast and can easily be coupled with other separation techniques e.g., liquid chromatography (LC). It is used to find out which molecules are present in the sample, their elementary composition, structure and quantity. The necessary parts of a mass spectrometer are an ion source, mass analyzer and detector (Hoffmann and Stroobant, 2007). Each mass analyzer has its advantages and limitations. Analyzers can be divided into two broad classes on the basis of many properties. Scanning analyzers transmit the ions of different masses successively along a time scale. They are either magnetic sector instruments with a flight tube in the magnetic field, allowing only the ions of a given mass-to-charge ratio to go through at a given time or quadrupole instruments. However, other analyzers allow the simultaneous transmission of all ions, such as the dispersive magnetic analyzer, the TOF mass analyzer, and the trapped-ion mass analyzers that correspond to the ion traps, the ion cyclotron resonance or the Orbitrap instruments (Hoffmann and Stroobant, 2007).
The GC-MS has been widely indicated as a “gold standard” for the identification of forensic bulks, as it is used to perform a specific test, which is a test that positively identifies the actual presence of a particular substance in a given sample. A non-specific test indicates that a substance falls into a group of substances. Sometimes, a non- specific test could lead to false positive identification by statistically suggesting the identity of the substance. As GC-MS associates the structures of gas chromatography and mass spectrometry to detect different materials within a sample, GC-MS has many applications including identification of any unknown samples, environmental analysis, drug detection, and explosives and fire investigation. GC- MS are also used in border security to detect substances on human beings or in luggage. Moreover, it is used to detect trace elements in materials that were previously thought to have disintegrated below detection limits. The GC-MS is composed of two major building units: the gas chromatograph and the mass spectrometer. The gas chromatograph employs a capillary column, whose analysis depends on the column’s dimensions, length, diameter, film thickness, as well as phase properties. In a mixture have different molecules, which have different chemical properties, the molecules will separate as the sample travels through the column. The retention time is the time taken by a molecule to come out of elute from the gas chromatograph. Since different molecules have different retention times, this allows the MS downstream to detect molecules separately. These molecules will be captured, ionized, accelerated, deflected, and detected separately. All these steps are done by the MS by fragmenting each molecule into ionized fragments and sensing these fragments using their m/z. The GC- MS units, used together, allow a much better-quality degree of identification of a substance than using each unit separately, i.e., it is unlikely to make a correct identification of a particular molecule by GC or MS alone. GC with a traditional detector, e.g., Flame Ionization Detector, can detect multiple co-elute molecules, that take place to have. In MS, two different molecules may have a similar pattern of ionized fragments.
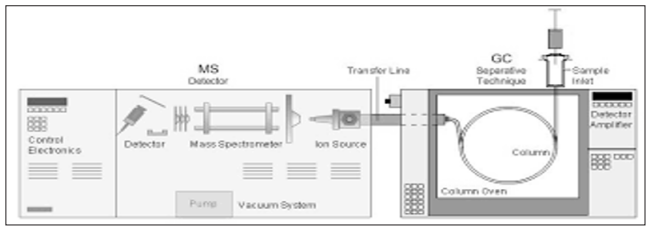
Figure 2: GC-MS Schematic (www.semanticscholar.org)
Combining the two methods makes it extremely improbable that two different molecules will behave in the same way in both a gas chromatograph and an MS, i.e., having the same retention time and a similar pattern of ionized fragments.

Figure 3: GS-MS/MS in CFS labs
Tandem mass spectrometry, MS/MS, is a more powerful technique to analyze quantitatively low levels of molecules of interest in the presence of a high sample matrix background. This is achieved by adding a second stage of mass fragmentation which is called MS/MS or Tandem MS. GC-MS/MS is ideal for chemical analysis and has been used for the analysis of target compounds in numerous matrices such as pesticides in foodstuffs, drug residues in biological fluids, environmental samples, drug metabolites, and other forensic trace evidence, it is used also to separate the organic compounds in the mixture. MS/MS is a highly selective mass spectrometric technique, target analytes are detected easily using MS/MS, regardless of the sample matrix or coeluting interferences. This technique is becoming more and more the analytical tool of choice when analyzing target compounds in highly complex matrices, where matrix is virtually eliminated in many GC-MS/ MS applications.
After separation by Gas Chromatography, the first stage of MS/ MS is to separate the ions from the matrix, where the MS/MS starts selecting the target ion(s) of choice at a specific mass. The MS detector measures m/z of ions using magnetic or electric fields which modify the movement of the ions letting the ions be arranged based on their mass. The ion current will be amplified and measured by an MS detector, and then the amount of sorted ion is quantitated. If tandem MS detectors are used, ion molecules with a specific m/z undergo additional fragmenting by a fragmentation process. These fragmented ions can then be detected by the second MS unit. These selected ions are known as precursor, or parent, ions. The resultant spectrum provides confirmation of the target analyte as it is a unique ion spectrum of the product. This improved MS/MS selectivity also boosts the signal to noise; therefore, lower limits of detection are achieved. GC-MS/MS affords clear identification in cases where GC-MS spectra of compounds are hard to realize. In order to improve detection levels and overcome the interference of pyrolysis product, MS/MS can be employed as the method of detection. It is very improbable that the interfering ion would yield the same daughter ion spectra as the analyte, even if the matrix encloses another compound with the same mass as the precursor ion for the analyte of interest (Hoffmann and Stroobant, 2007).
High-Pressure Liquid Chromatography (HPLC) Chromatographic techniques are used for the separation of components, due to their different affinity to the stationary and mobile phase. HPLC stands for high-pressure (also high- performance) liquid chromatography and uses a liquid mobile and a solid stationary phase. It is a commonly used technique for the separation and quantification of molecules. The apparatus consists of a pump, an injector, a column and a detector. High pressure in HPLC technique is used to push samples through a chromatography column to separate out the molecules. The method of separating the molecules depends on the type of chromatography column used. There are several different types of columns size exclusion, ion exchange, normal phase and reverse phase columns. Once the molecules pass through the column, they will be detected by some sort of detector. Typically, this detector is a UV detector or PDA, but other detectors such as refractive index detectors, laser light scattering detectors, fluorescence detectors, thermal conductivity detectors, and MS can also be used. When using HPLC there are two phases: the mobile phase, also called the eluent, and the stationary phase. The mobile phase is the liquid that transfers the samples through the instrument and through the columns. Water, methanol, and acetonitrile are examples of the mobile phase. In the stationary phase, which takes place in the column that the sample passes through, molecules are separated by the speed at which they are able to pass through the column. For instance, in size exclusion chromatography columns, larger molecules are too big to enter pores within the column, thus they pass straight through the column and elute more quickly than small molecules, which take a longer route through the column because they enter into pores within the column. The chromatograph resulted from the retention time and the analysis from the detector that should be evaluated (Hoffmann and Stroobant, 2007).

Figure 4: HPLC-PDA in CFS labs
Variable wavelength detectors can be tuned to operate at the absorbance maximum of an analyte or at a wavelength that provides more selectivity. They can also be programmed to change wavelengths during a chromatographic run to compensate for the response of different analytes. In a variable wavelength detector, light from a broad spectrum (for UV deuterium is common, tungsten for visible) lamp is directed through a slit to a diffraction grating that spreads the light out into its constituent wavelengths. The grating is then rotated to direct a single wavelength of light through a slit, through the detector cell, to a photodiode. An example schematic for a variable wavelength detector is shown in Fig. (5). PDA has an optical path similar to variable wavelength detectors except the light passes through the flow cell prior to hitting the grating, allowing it to spread the spectrum across an array of photodiodes, as illustrated in Fig. (6).
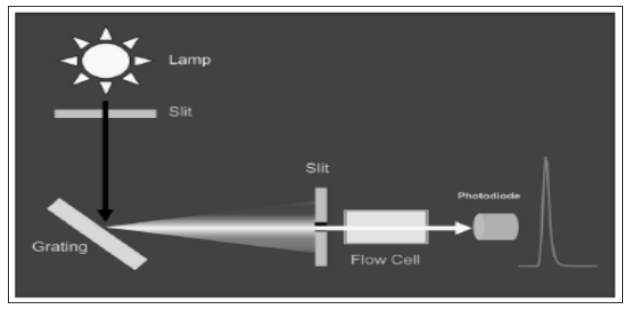
Figure 5: Variable wavelength UV detector schematic (Swartz,2010)
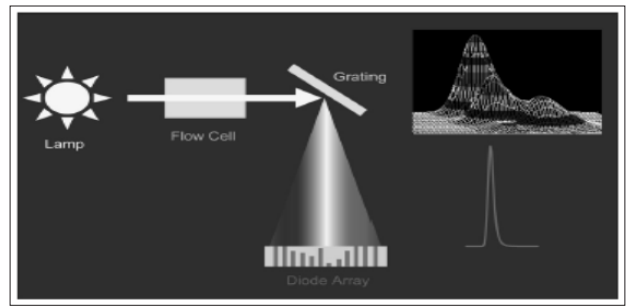
Figure 6: PDA detector schematic (Swartz, 2010)
The new PDA detector cell type consists of a light-guided flow cell equivalent to an optical fiber. Light is efficiently transferred down the flow cell in an internal reflectance mode that still maintains a proper flow cell path length with a proper volume; extending the path length while maintaining low dispersion. Light-guided flow cells are particularly useful in maintaining low dispersion in HPLC systems. PDAs extend the utility of UV detection by providing spectra of eluting peaks that can be used to aid in peak identification and to monitor for co-elution (peak homogeneity or purity), helpful during method development. They can also serve as a multi-wavelength UV/VIS detector. The spectra collected at the chromatographic peak apex can be used to create a library that can in turn be used to compare subsequent spectra for identification purposes, and spectra collected across the peak at each data point can be compared to evaluate peak homogeneity or purity (Swartz, 2010).

Figure 7: LC-MS/MS in Research Labs
LC-MS/MS is one of the most common bioanalytical methods in use today. This is a very flexible, robust, and sensitive methodology that is used for nearly all small molecules. In addition, this technology is responsive to automation and unattended analysis.
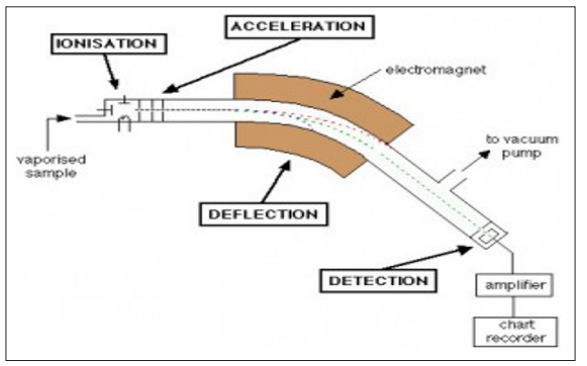
Figure 8: LC-MS Schematic from (www.certara.com)
LC-MS instruments are principally HPLC units with a mass spectrometry detector attached to it whereas LC-MS/MS is HPLC with tandem mass spectrometry. The liquid chromatography part of LC-MS separates compounds within a sample and the MS provides m/z data which can help provide structural identity of the compound. Applications of LC-MS range from food analysis, environmental testing, drug development work, and medical products testing.
There are some differences between HPLC trials and those of
LC-MS:
Molecules in the chromatography column are under high pressure, and when they are eluted from the column, an interface is required between the end of the column and the beginning of the MS. This is because MS units operate in a vacuum, thus, the continuous flow eluted from the column cannot be directly detected by the MS. The interface eliminates the chromatography mobile phase and transfers the analyte to the MS unit. In the interfaces, the liquid is nebulized into a fine spray, ionized and then transferred to the MS detector.
The MS detector transfers the m/z data to a computer which presents the information as a mass spectrum graphically. The mass spectrum of the sample can be used to:
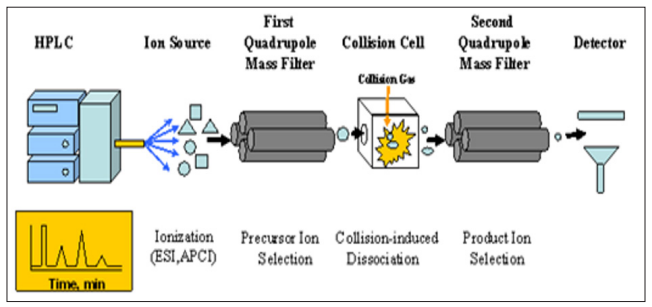
Figure 9: LC-MS/MS Schematic (from www.toray-research.co.jp)
When a single ionization is not satisfactory to attain the desired sensitivity, the MS/MS tools are used. Controlling the ionization process is the key challenge in MS detection. Incomplete ionization and interference lead to poor precision and accuracy, which can be resolved with numerous different ionization techniques, solvents, and even chromatographic separations.

Figure 10: LC-QTO
The concept of time-of-flight (TOF) analyzers was described by Stephens in 1946. The design of a linear TOF mass spectrometer was published by Wiley and McLaren in 1955, this later became the first commercial instrument, and TOF popularity was restricted until the 1990s. Recent improvements in TOF technology, including ion mirrors (reflectron), orthogonal acceleration, and high-speed electronics, have significantly improved TOF resolution. Fig. (11) displays the scheme of a linear TOF instrument.
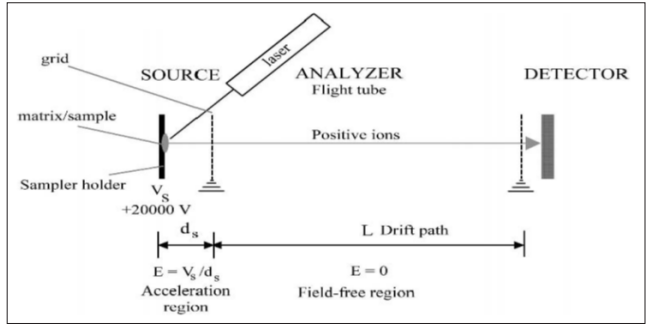
Figure 11: Principle of an LTOF Instrument Tuned (Hoffmann and Stroobant, 2007)
Since the end of the 1980s, and due to the following reasons, there has been renewed interest in these instruments:
The TOF analyzer separates ions, according to their velocities
when they drift in the flight tube.
An additional motivating characteristic of the TOF analyzer is its easy mass calibration with only double reference points. The physical property that is measured during an analysis is the flight time of the ions. A calibration equation is required to relate and transform the measured physical property to a mass value, as in all the mass spectrometers. Increasing the resolution of TOF analyzers could be achieved by lengthening the flight tube since the mass resolution is proportional to the flight period and the flight track. However, too extended flight tube decreases the performance of TOF analyzers due to the loss of ions which occurs by scattering after collisions with ambient molecules or by spreading of the ion beam. Lowering the acceleration voltage leads to an increase in the flight time. But this will reduce the sensitivity. Therefore, the only way to have optimal high resolution and high sensitivity is to use a 1 to 2 m length flight tube with at least 20 kV acceleration voltage to keep the sensitivity high.
Quadrupole Time-of-Flight Mass Spectrometry (Q-TOF) The most successful type of hybrid instrument in which TOF has been integrated combines a quadrupole analyzer with a TOF instrument in a Q-TOF configuration. These instruments are powerful and robust with unique performances. They give high sensitivity in the attomole range (E-18). The rapid success of this type of hybrid instrument is due to the attractive combination of the simplicity of the quadrupole and the high performance of the TOF. As shown in Fig. (12), the most common of these instruments include a quadrupole analyzer Q1 and a quadrupolar collision cell q2, followed by an oa-TOF. They thus have the QqTOF configuration. This instrument can be described as a triple quadrupole where the last quadrupole is replaced by an oa-TOF, or as the addition of a quadrupole analyzer and a collision cell to a TOF analyzer.
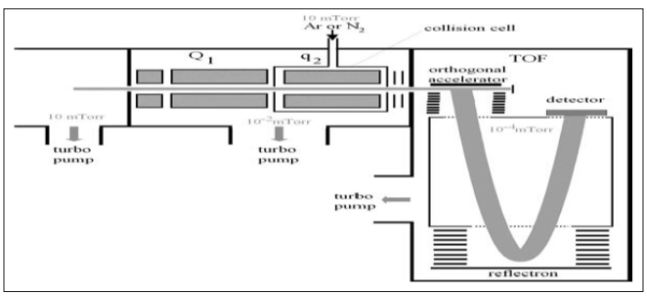
Figure 12: Scheme of a hybrid mass spectrometer including a quadrupole analyzer, a quadrupolar collision cell, and an orthogonal acceleration time-of-flight analyzer (Hoffmann and Stroobant, 2007). The TOF analyses all the ions that have been orthogonally accelerated and acts as the only mass analyzer. The resulting spectra support the good performances of the TOF analyzer regarding resolution and mass accuracy but with a mass range limited by the transmission of the quadrupoles. The q2 quadrupole may or not contain a collision gas. If it contains a gas at sufficiently low pressure and the ions have a low kinetic energy, the energy transmitted will remain lower than the fragmentation threshold but the ions will lose kinetic energy in the radial and axial directions. This will improve the resolution and the sensitivity in the TOF analyser by reducing the flight time distribution of ions with a given mass value. Altogether in these instruments, the velocity spread of the ions is strongly reduced. Furthermore, the beam is strongly focused in the axial direction, thus also reducing the spatial spread at injection time. The beam is then much better prepared for injection in the TOF than those issued from a directly connected ion source.
Combating falsified medicines become a priority issue that involves many players. To characterize falsified samples chemically, and evaluate their hazards for patients, analytical techniques have many contributions by performing proper analyses. A widespread collection of techniques is used to achieve individual information on the medicine’s composition, the presence of active material or impurities, or the crystalline arrangement of their formulations. An approach is presented in the review paper by [9]. in which they put forward a methodology to combine analytical techniques. In order to illustrate this approach, examples from scientific literature on erectile dysfunction treatment, weight loss, and malaria, are placed in the center of the proposed methodology. They concluded that combining analytical techniques allows the analyst to decide on the falsification of a sample, its compliance with pharmaceutical quality, and its safety for patients. Many studies are conducted on different types of medications [9-19]. The abuse or misuse of fake medications is a serious global problem. So, the detection of genuine medication and related active analogues in counterfeit drugs or the differentiation between counterfeit and authentic, has been performed with a variety of analytical methods. The seriousness of the drug counterfeiting issue is enhanced by the industrialization and globalization of the phenomenon. Bearing in mind the serious consequences and dangers related to counterfeit drugs as well as the steadily growing migration of them all over the world, new, relatively simple, and effective methods that can support the detection of counterfeit drugs as well as certain strategies are strongly desired. Due to the large diversity in sample types and pharmaceutical product of concern in different regions, analysis methods can vary significantly in their choice of target pharmaceutical products and transformation products and this makes it challenging for an analyst to select a method for analysis as they may not fully understand the factors that went into the selection of the instrumental parameters and the compromises that were made to resolve matrix effects, chromatographic needs, and detection requirements.
In this paper a comparison between different analytical techniques has been done, where five analytical techniques were investigated as the most efficient technique to analyze genuine and generic drugs, since several genuine and generic are classified as counterfeits and vice versa. Combining the analytical results provides a comprehensive characterization of the falsified medicinal products. This information is essential for patient safety and to help authorities in their task of combatting falsified products. Analytical techniques have been considered in this study are: UV-Vis, GC-MS/MS, LC-DAD, LC-MS/MS and LC–TOF/
MS instruments. With modern counterfeiting techniques growing more sophisticated, it has become essential for every organization involved in the pharmaceutical supply chain, from international authorities to the manufacturers themselves, to arm themselves with the right methodology, analytical techniques and equipment to tackle this problem head on.
