Author(s): Tarit Kanti Ghosh
Parkinson’s disease (PD) is recognised as the second most commonly occurring neurodegenerative disorder, after Alzheimer’s disease. As per the Parkinson Disease Foundation, it is estimated that Parkinson’s disease affects around 7-10 million people worldwide, as reported by de Souza et al. The principal aetiology of PD is the degeneration of neurons that produce dopamine in the brain. Dopamine is a crucial neurotransmitter that regulates the smooth and coordinated functioning of muscles. The primary motor manifestations of PD comprise resting tremor, bradykinesia, rigidity, and compromised postural stability
The identification of PD can pose a challenge, particularly during its initial phases. Presently, there are no definitive assessments or biomarkers that can be employed to diagnose PD. According to the study of Goetz et al the present diagnostic approach primarily relies on subjective evaluations conducted by clinicians and neurologists, who utilise visual observations to generate a score based on the “Unified Parkinson’s Disease Rating Scale (UPDRS)” [1]. In general, a neurologist conducts a comprehensive review of the patient’s medical records and conducts multiple clinical evaluations to establish the existence of PD in the individual [2]. In some instances, the diagnosis of Parkinson’s disease may necessitate a duration of up to 12 months, during which a comprehensive assessment of the patient’s neurological history and clinical evaluations are performed.
Furthermore, the absence of standardised criteria for assessment increases the likelihood of erroneous identification of PD. Research has indicated that there exists a misdiagnosis rate of approximately 25% for PD, and roughly 40% of instances of PD are disregarded in favour of alternative neurological conditions [3]. As per the analysis of professionals, the identification of PD necessitates the existence of at least one of the four principal motor symptoms associated with PD.
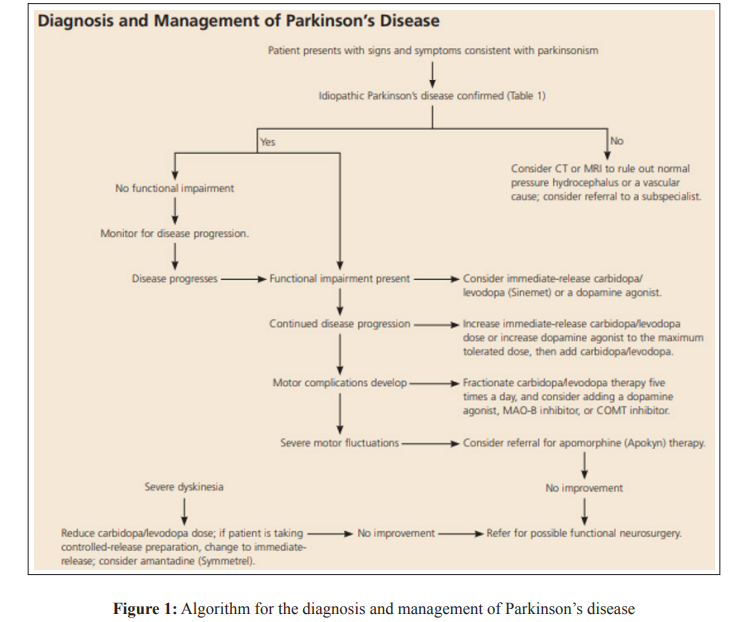
The manifestation of PD symptoms exhibits interindividual variability. As per the study of Espay et al, during the onset of PD, it has been observed that resting tremor manifests in only 70% of patients, whereas some individuals may experience gait disturbances or action tremor as initial symptoms [4]. The timely and accurate identification of Parkinson’s disease is essential for the most favourable medical intervention and efficient handling of symptoms, as stated by Zach et al [5]. Customising the therapeutic approach to the individual’s requirements is of utmost importance in diminishing indications and alleviating both physical and non-physical issues, as depicted in Figure 1. As the pathology advances, the therapeutic approach may become progressively intricate, necessitating the involvement of a subspecialist for co-management.
Over the course of time, various research methodologies have been developed to create a monitoring system for PD. These methodologies employ diverse sensor types, feature sets, and analysis techniques. Al-Otaibi et al highlighted in their study that, Accelerometers, force sensors, gyroscopes, and magnetometers are among the wearable sensors commonly utilised for obtaining bio-signals [6]. The study conducted by Patel et al. aimed to create a wearable sensor platform that can accurately quantify the severity of tremor, bradykinesia, and dyskinesia, which are motor symptoms associated with PD.
The algorithm proposed by Belic et al aimed to identify and assess tremor, and subsequently established a comparison between the quantified tremor amplitude and the corresponding UPDRS score [7]. Landers et al mentioned in their research that, employed tremor features, including amplitude, frequency, and spectral power, to discern PD tremor. Subsequently, the amalgamation of said characteristics was executed to form a solitary variable, thereby enabling the efficient identification of a PD from an atypical tremor [8]. Furthermore, it is crucial to observe the gait abnormalities in individuals in order to identify PD in its initial phase.
Tarakad and Jankovic, conducted experiments and deduced that Parkinsonian patients exhibited lower stride velocity, stride length, and swing time as compared to healthy control subjects. Conversely, the duration of stance phase in individuals with PD was found to be greater in comparison to that of individuals without the condition [9,10]. Furthermore, Langston et al achieved comparable outcomes through the utilisation of a force sensor that was worn by the participants [11]. In addition, Schrag et al conducted an extraction of basic, kinetic, and kinematic features utilising force measurement [12].
Subsequently, a statistical analysis was conducted and it was determined that step length, walking speed, and Vertical Ground Reaction Force (VGRF) were notable characteristics that could distinguish an individual with PD from a healthy control participant [13]. Barth and colleagues extracted a range of gait features and subsequently applied multiple classifiers to classify them. The individual performances of these classifiers were then analysed. Of the classifiers employed, LDA (Linear Discriminant Analysis) yielded the highest classification accuracy. Bhoi, investigated the correlation between gait variability and walking speed in individuals with PD and a healthy control group [14].
The most distinctive characteristic of PD is bradykinesia, which refers to a reduction in movement speed. The clinical presentation of bradykinesia may include hyperkinesia, which refers to the presence of small amplitude movements, as well as akinesia, which is characterised by a paucity or absence of movements. Sage found that the first signs of this condition may present as a reduction in facial expressions such as blinking, which is also regarded as hypomania, difficulty with fine motor control, excessive salivation or drooling due to impaired swallowing, slow performance of daily tasks such as reduced arm swing while walking [15].
It is crucial to conduct screening for alternative factors that may contribute to decelerated movement, such as reduced strength ordecreased motivation (which may manifest in cases of depression). During clinical evaluation, it is important to comprehensively evaluate bradykinesia by observing the performance of repetitive movements, including opening and closing of hands, at maximum speed [16]. The objective is to evaluate the presence of slowness and decrementing amplitude. It is crucial to consider supplementary indicators of bradykinesia, such as non-verbal communication, facial gestures, eye blinking, in both the anamnesis and clinical evaluation.
The hallmark tremor associated with PD is a supination-pronation hand tremor that exhibits a frequency of 4 to 6 Hz and is commonly referred to as a “pill rolling” tremor. This tremor is typically observed during periods of rest. According to Thenganatt and Jankovic, it is crucial to distinguish between the classic rest tremor and the primarily action tremor, which may be present in individuals with Parkinson’s disease but is more commonly associated with Essential Tremor (ET) [17].
However, if the patient displays tremors in the head, voice, or handwriting, it may be necessary to consider the possibility of ET either on its own or in conjunction with PD. The temporal association between ET and PD remains unclear, despite some patients experiencing ET for extended periods before the onset of PD [18]. The pathogenic link between these two conditions requires further investigation. Gallagher et al. found that individuals diagnosed with Parkinson’s disease commonly report experiencing “internal tremor,” a tremulous sensation that is not externally visible, in addition to rest and postural tremor. Research findings indicate that a considerable proportion of individuals diagnosed with Parkinson’s disease, ranging from 31% to 44%, have reported experiencing this particular symptom.
To assess tremor, it is necessary to observe the tremor at rest while the limbs are in a relaxed position, as well as during ambulation. Furthermore, an assessment of postural tremor can be performed by executing exercises such as extending the arms in a horizontal orientation anterior to the body and in a position resembling the flapping of wings [19]. Kinetic tremor can be assessed through exercises such as finger-to-nose testing, as outlined by SanchezFernandez et al. [19]. A significant proportion of patients who display rest tremor also exhibit re-emergent tremor. This is a condition where the postural tremor does not appear for several seconds or minutes after adopting an antigravity posture, such as holding the arms outstretched in front of the body or in a wingbeating position.
The frequency similarity observed between the re-emergent tremor and the rest tremor in individuals diagnosed with Parkinson’s disease serves as evidence supporting the pathophysiological connection between the two forms of tremors. In their study, Tarakad and Jankovic noted that supinating-pronating or reemergent tremor is frequently observed in patients while walking, and is often accompanied by a significant worsening of the rest tremor [9]. Maintaining a attentive observation of the patient during the history and examination process is crucial in accurately distinguishing rest and other tremors associated with Parkinson’s disease from those of essential tremor or other types of tremors.
Postural instability is commonly observed in the later stages of PD, and is recognised as one of the four primary motor symptoms of the condition. The instability observed can be attributed to the absence of automatic postural reactions triggered by visual, vestibular, and somatosensory inputs, as well as the lack of anticipatory postural adjustments that are typically employed to counteract expected postural disturbances prior to their occurrence [20]. Additional factors that may potentially contribute to postural instability include orthostatic hypotension, freezing, and fear of falling. The assessment of postural instability can be effectively executed by means of the pull test as part of a clinical examination. This exercise entails the swift and forceful backward displacement of a patient from their shoulders.
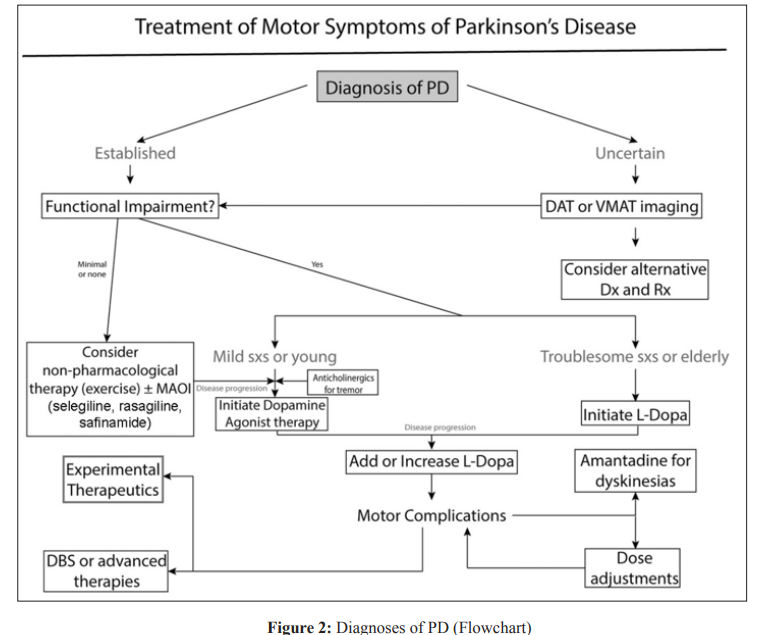
The comprehensive management of Parkinson’s disease involves addressing a range of factors, including the management of both the motor and non-motor symptoms of the disease, across its various stages, from early to advanced. After the confirmation of PD, a fundamental algorithm ([Fig. 2]) can guide the determination of when and how to proceed with treatment. However, it is crucial to underscore that the therapy for PD must be customised and adapted to the particular requirements of each patient. The pharmacological treatments are commonly used in addition to non-pharmacological interventions such as relaxation techniques, for mild symptoms. These treatments include anticholinergic medications, dopamine agonists, and monoamine oxidase inhibitors (such as safinamide, selegiline, and rasagiline) [9]. The initiation of levodopa therapy typically occurs when symptoms become problematic and start to impede daily living or occupational functioning.
As the disease progresses and motor complications such as motor fluctuations and dyskinesias emerge, it is recommended to consider surgical and experimental therapeutic options. Bloem et al stated that, despite the absence of efficacious neuroprotective interventions, a number of clinical trials are presently underway with the aim of devising plausible disease-modifying approaches [21]. In addition to inosine, which has been shown to increase urate levels, and isradipine, a medication that blocks calcium channels, there are other substances and drugs that may have an impact on urate levels. There also exist therapeutic interventions aimed at impeding the aggregation or facilitating the clearance of hazardous α-synuclein. In the absence of evidence demonstrating the efficacy of various therapies in impeding or halting the advancement of the disease, a rigorous exercise regimen is presently advocated as the optimal strategy aimed at potentially exerting a beneficial impact on the progression of the disease [22]. In relation to this topic, it has been found that engaging in physical activity and therapy, particularly through resistance and endurance training and other intensive training techniques, can yield the greatest benefits.
Levodopa therapy has been regarded as the standard of care since its inception in the 1960s. Across the annals of time, a plethora of iterations of levodopa therapy formulations have been devised. Tambasco et al. conducted a study which revealed that Levodopa is commonly administered in conjunction with a peripheral decarboxylase inhibitor, such as carbidopa or benserazide [23]. This is done to impede the conversion of levodopa to dopamine in the periphery and forestall the occurrence of nausea. The management of patients undergoing levodopa therapy poses a significant challenge for clinicians. The main aim is to sustain the patient’s relief from symptoms or “ON” time while concurrently averting the emergence of peak-dose dyskinesia, which usually manifests as stereotypy (e.g., head bobbing), chorea, dystonia, or myoclonus. Moreover, clinicians endeavour to reduce the frequency of wearing-off phenomena, which pertain to the resurgence of symptoms associated with Parkinson’s disease.
The correlation between the display of motor complications associated with levodopa and the duration and dosage of levodopa therapy, as well as the underlying illness duration, has been observed. Verschuur et al. conducted a study which revealed that individuals who receive a diagnosis of Parkinson’s disease at a younger age are more vulnerable to complications associated with the condition than those who receive a diagnosis later in life [24]. In order to effectively manage motor complications, it is frequently required to reduce the individual dosage of medication and concurrently augment the frequency of administration.
Numerous formulations of levodopa have been devised to enhance the pharmacokinetic properties of the medication. Tambasco et al reported that Sinemet CR (Merck Co.), which was developed in the 1980s, is the initial extended-release formulation of levodopa that has been discovered to be efficacious in extending the duration of levodopa’s therapeutic effects [23]. However, this phenomenon is linked to irregular absorption, inadequate or delayed response, and exacerbation of peak-dose dyskinesias. The emergence of a novel prolonged-release iteration of carbidopa-levodopa, referred to as Rytary and formulated by Impax Laboratories, Inc., has been noted to diminish motor fluctuations and dyskinesias, while preserving the duration of “on” time. Duopa is a levodopa medication that is administered in the form of an intestinal gel. The main aim of this medication is to mitigate the variations in levodopa concentrations by ensuring continuous administration via an enteric pump.
Standaert et al. conducted a clinical investigation spanning 12 weeks, wherein 66 patients diagnosed with advanced Parkinson’s disease and motor complications were randomly assigned to either an intestinal gel group or an immediate-release oral group [25]. The research was carried out utilising a methodology that incorporated both double-blind and double-dummy designs. The study conducted by Virhammar and Nyholm revealed that the administration of intestinal gel resulted in a reduction of 4.04 hours (± 0.65) in the average duration of “off” time [26]. In contrast, the group that received immediate-release oral medication experienced a reduction of 2.14 hours (± 0.66). The results indicate a statistically significant disparity of -1.91 hours (p=0.0015) between the two groups. As per the findings of the study, themean duration of dyskinesia-free period was observed to have increased by 4.11 hours (± 0.75) in the cohort treated with intestinal gel, while the group administered with immediate-release oral medication exhibited an increase of 2.24 hours (± 0.76).
The observed difference between the two groups was 1.86 hours, and the p-value was 0.0059 [27]. The aforementioned therapeutic approach, while exhibiting significant efficacy in ameliorating levodopa-induced complications, is nevertheless linked to a notable incidence of complications, particularly those pertaining to the jejunostomy tube. Recent advancements in therapeutic interventions involve the utilisation of inhaled levodopa (CVT301; Acorda Therapeutics) and transdermal formulations. These treatment methods have demonstrated potential in efficiently addressing motor fluctuations in a prompt manner.
The efficacy of dopamine agonists as a monotherapy for patients with Parkinson’s disease of mild to moderate severity has been demonstrated. The prioritisation of these medications as the primary treatment option for younger patients is a common practise in clinical settings. The objective of this methodology is to defer the commencement of levodopa treatment and mitigate the possibility of dyskinesia caused by levodopa. According to a study conducted by Yu et al. the transdermal administration of dopamine agonists, including ropinirole, pramipexole, and rotigotine, is a commonly employed technique in the US [28]. The discontinuation of older ergot dopamine agonists, namely pergolide and bromocriptine, has been attributed to the augmented likelihood of valvular, pulmonary, and other associated complications. The subcutaneous injection of Apomorphine is a therapeutic modality that entails the stimulation of dopamine receptors. The medication exhibits a rapid onset of action and may be employed intermittently to alleviate instances of unforeseen diminution in medication efficacy experienced by patients.
In addition, the administration of the aforementioned treatment modality through a continuous subcutaneous infusion is a viable option for establishing a means of sustaining therapy. Cerri and Blandini have reported that the co-administration of dopamine agonists with levodopa treatment has been observed to enhance and prolong its therapeutic effects [29]. The adverse effects that are linked with dopamine agonists exhibit similarities to those of levodopa. However, it is widely acknowledged that dopamine agonists are linked to orthostatic hypotension, hallucinations, confusion, somnolence, leg edoema, and impulse control disorders. Hence, it is recommended to exercise caution while dispensing them to geriatric or cognitively challenged individuals.
The inhibition of Catechol-O-methyltransferase has the potential to obstruct the peripheral metabolism of levodopa, thereby prolonging its duration and augmenting its bioavailability. The aforementioned intervention has demonstrated efficacy in ameliorating off time and augmenting on time in individuals with moderate to severe PD [30]. However, it is noteworthy that its administration has been associated with an elevated likelihood of dyskinesias. The inhibition of dopamine metabolism is the mechanism by which selective monoamine oxidase B inhibitors increase the activity of dopaminergic neurons in the striatum.
Despite the fact that they have demonstrated efficacy in ameliorating motor fluctuations, their predominant employment occurs during the initial stages of the ailment, when the indications are comparatively moderate, and as tactics for postponing the administration of levodopa. Research by Silva et al, investigating the possible neuroprotective or disease-modifying impacts of these pharmaceuticals has generated contradictory and inconclusive findings [31]. Amantadine has been found to be efficacious in the treatment of tremor and bradykinesia associated with PD. However, its predominant use is in the management of dyskinesias induced by levodopa. The administration of stimulants, such as methylphenidate and atomoxetine, may potentially serve as a viable treatment option for freezing of gait, a condition that may otherwise prove challenging to manage.
PD is a prevalent and intricate condition that poses challenges in both its identification and treatment. A comprehensive comprehension of concomitant manifestations, along with uncharacteristic manifestations, is crucial for a precise diagnosis. Levodopa therapy is considered the foremost intervention for Parkinson’s disease, particularly in instances of progressed or severe illness, due to its high efficacy. Nevertheless, there exist a number of alternative pharmaceutical interventions that may serve as adjunctive therapy or be administered during the initial phases of the ailment. Contemporary investigations are examining innovative surgical interventions that serve a twofold objective of mitigating symptoms in individuals with progressive Parkinson’s disease and conceivably modifying the trajectory of the ailment. The field of experimental therapeutics in PD offers promising prospects for the development of improved therapies that are both safer and more effective. By focusing on critical pathogenic mechanisms, these therapies have the potential to not only enhance the quality of life for PD patients, but also to potentially impede or halt the progression of the disease [32].
