Author(s): Nardine Abdelsayed*, Larissa Check, Chadley Froes, Jillian Sansbury, Brendon Cornett and Andrew Mangano
According to the 2009 AHA/ASA scientific definition, a transient ischemic attack (TIA) is defined as a transient episode of cerebral, spinal cord or retinal ischemia without evidence of acute infarction on imaging [1]. Most patients who present with TIA are effectively managed similarly to stroke patients, unless presenting symptoms completely resolve at the time of admission, thus raising suspicion for TIA. An episode of TIA is classically associated with an increased risk for future cerebrovascular accident (CVA), although recent meta-analysis found a reduced association over the last two decades [2]. When evaluating a patient with suspected TIA, scoring systems such as the Canadian ABCD2 score is often performed as risk stratification, or to predict which patients have a higher risk of stroke within 48 hours [3]. Despite routine utilization, such risk stratification systems may have little bearing on management, and the standard of care for workup of TIA/CVA almost always includes immediate determination of the patient’s last known well status, NIH stroke scale scoring, and emergent computerized tomography (CT) neuroimaging. This is typically followed by a 24-hour period of permissive hypertension during which additional imaging is pursued to identify pathologic processes that may be identified as etiology for CVA, including carotid patency studies (i.e., doppler ultrasound or CT angiography), brain MRI, and transthoracic echocardiography (TTE), in addition to electrocardiography (ECG) and other forms of continuous cardiac monitoring [1,3]. This facilitates the identification and treatment of underlying causes of cardioembolic phenomenon using antiarrhythmic and antiplatelet agents, and serves as the foundation for secondary prevention alongside rigid glycemic control, blood pressure control, and smoking cessation.
Interestingly, although current guidelines from the AHA/ASA recommend TTE when cardioembolic source is suspected, there is less clarity regarding the specifics of patient selection, or what should be done with the results. Cardioembolic stroke accounts for up to 25% of all ischemic CVA [4]. Most cases are associated with atrial fibrillation (AF), but less common causes include left ventricular thrombi, prosthetic valves, cardiac tumors, and bacterial endocarditis [5]. The pathogenesis of cardioembolic strokes in atrial fibrillation is understood to occur due to intravascular stasis with intramural clot formation and subsequent thromboembolism. Generally speaking, the primary goal of TTE in the workup of CVA/TIA is to identify the underlying cardioembolic source of ischemia, particularly in cases of cryptogenic CVA. Previous studies have linked CVA to left atrial enlargement greater than 4 cm, but few have delineated the clinical utility of early TTE specifically in the setting of TIA, nor the value of prescribing outpatient cardiac monitoring at discharge for this select group of patients [6]. As such, our study aimed to determine which echocardiographic features are associated with TIA and to further specify the clinical utility of various findings noted during TTE.
We conducted a retrospective cohort study of patients admitted to the hospital with discharge diagnosis of TIA from 2018-2019. A total of 588 patients from the South Atlantic Division of Hospital Corporation of America (HCA) hospitals aged 18 and older were identified based on a confirmed discharge diagnosis of TIA. Patients were excluded if they had an acute stroke, demyelinating disease or intracranial mass on MRI of the brain or carotid stenosis greater than 50%, resulting in a total population of 588 patients. Of these, 139 patients were further excluded on the basis of stroke, lack of documented TTE findings, or carceral admission or discharge.
A total of 449 (76.36%) patients met the inclusion criteria and had a two-dimensional (2D) echocardiogram as part of their TIA evaluation. Of the 449 patients, TTE was performed with agitated saline in 130 patients (28.95%) and without agitated saline for 319 patients (71.05%). TTE reports were reviewed for the presence of findings including LA size greater than 4 cm, atrial fibrillation, left ventricular thrombus, LA thrombus, moderate to severe mitral stenosis, patent foramen ovale, atrial septal defect, vegetation or mass on the valve, and atrial myxoma. LA enlargement was the only finding with sufficient frequency to power statistical significance testing of baseline comorbidities.
Using the chi-squared test, all patients with LA enlargement (173) were further divided into those with AF and without AF. There were 59 (34.10%) patients with AF and 114 (69.10%) without AF. The groups were compared for differences in demographics, echocardiogram findings, as well as comorbidities. Using the Chi-Squared test, we also examined which patients with left atrial enlargement were offered a 30-day period of outpatient cardiac monitoring via cardiac monitoring device (i.e. loop recorder, event monitor).
The data collected on 588 patients aged 18 and older presenting with TIA from 2018-2019 were analyzed. Patients were excluded if they did not have an echocardiogram as part of their workup, had an acute stroke, demyelinating disease or intracranial mass on MRI of the brain, or carotid stenosis greater than 50%, with a final sample population of 449 patients. Features are noted in Table 1. Demographics of our population included a majority Caucasian population (84.41%). Various comorbidities were identified within our sample population, including hypertension (81.51%), dyslipidemia (58.13%), smoking history (49.44%) and diabetes mellitus (34.08%) as seen in Table 2.
| Demographics | Patients (N=449) |
|---|---|
| Age: | |
| Median (IQR) | 70 (60 - 79) |
| Range | 31 - 91 |
| Race: | |
| Asian | 1 (0.22%) |
| Black | 51 (11.36%) |
| Multiracial/Other | 17 (3.79%) |
| Native American | 1 (0.22%) |
| White | 379 (84.41%) |
| Sex: | |
| Female | 242 (53.90%) |
| Male | 205 (45.66%) |
| BMI: | |
| Median (IQR) | 28 (24 - 33) |
| Range | 16 - 61 |
| Comorbidities | All Patients (N=449) |
|---|---|
| Coronary Artery Disease | 108 (24.05%) |
| Dyslipidemia | 261 (58.13%) |
| Atrial Fibrillation | 80 (17.82%) |
| Heart Failure | 55 (12.25%) |
| Hypertension | 366 (81.51%) |
| Cardiac Insufficiency | 8 (1.78%) |
| Peripheral Vascular Disease | 15 (3.34%) |
| Diabetes | 153 (34.08%) |
| Chronic Kidney Disease | 77 (17.15%) |
| ESRD On Dialysis | 5 (1.11%) |
| COPD | 49 (10.91%) |
| Alcohol Use Disorder | 16 (3.56%) |
| Tobacco Use Disorder | 222 (49.44%) |
The incidence of mitral stenosis, PFO, atrial septal defect, left atrial thrombus, atrial myxoma and vegetation was too low to perform further statistical analysis (Figure: 1). PFO was discovered in 11 of the 130 (8.46%) TTEs performed with agitated saline, and none were discovered in those performed without agitated saline. Furthermore, 173 of the 449 (38.53%) patients were found to have a LA enlargement greater than 4 cm. Amongst these patients, 59 of the 173 (34.10%) had a known diagnosis of atrial fibrillation or new diagnosis of atrial fibrillation during the admission.
| Echocardiographic Findings | TTE with agitated saline (N=130) |
TTE with agitated saline (N=319) | All Patients (N=449) |
|---|---|---|---|
| Left Atrium Enlargement > 4.0 cm | 47 (36.15%) | 126 (39.50%) | 173 (38.53%) |
| Moderate/Severe Mitral Stenosis | 1 (0.77%) | 1 (0.31%) | 2 (0.45%) |
| Left Atrial Thrombus | 1 (0.77%) | 1 (0.31%) | 2 (0.45%) |
| Patent Foramen Ovale | 11 (8.46%) | 0 (0.00%) | 11 (2.45%) |
| Atrial Septal Defect | 5 (3.85%) | 6 (1.88%) | 11 (2.45%) |
| Vegetation/Mass, Aortic or Mitral Valve | 0 (0.00%) | 2 (0.63%) | 2 (0.45%) |
| Atrial Fibrillation | 7 (5.38%) | 23 (7.21%) | 30 (6.68%) |
| New EF Less Than 50% | 9 (6.92%) | 23 (7.21%) | 32 (7.13%) |
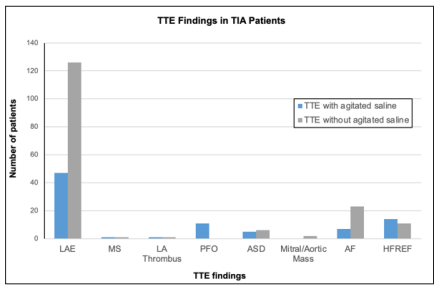
Figure 1: Comparing Pertinent TTE Findings in Patients with TIA who Underwent TTE Versus those without Aagitated Saline. (LAE: left atrial Enlargement < 4cm; MS: Mitral Stenosis; PFO: Patent Foramen Ovale; ASD: Atrial Septal Defect, AF: Atrial Fibrillation; HFREF: Heart Failure with New Reduced Ejection Fraction < 50%)
Frequency of TTE findings including LA enlargement > 4 cm, mitral regurgitation, left ventricular thrombus, patent foramen ovale, atrial septal defect, aortic or mitral vegetation was further stratified based on the presence or absence of atrial fibrillation. Using chi-squared analysis, new EF less than 50% was statistically more likely in patients with AF as opposed to those without (23.73% vs 9.65%, p=0.013). Chi-squared analysis revealed a statistically significant difference in the frequency of heart failure (44.07% vs 15.79%, p<0.001) and chronic kidney disease (38.98% vs 20.18%, p=0.008) in patients with AF when compared to those without AF, as shown in Table 4. Amongst the patients who had a left atrial enlargement greater than 4 cm and no prior history of atrial fibrillation, only 6 out of 114 patients (5.26%) were referred for outpatient cardiac monitoring.
| Comorbidities | Atrial Fibrillation (N=59) |
No Atrial Fibrillation (N=114) |
All Patients (N=173) | p-Value |
|---|---|---|---|---|
| Coronary Artery Disease | 24 (40.68%) | 37 (32.46%) | 61 (35.26%) | 0.283# |
| Dyslipidemia | 39 (66.10%) | 71 (62.28%) | 110 (63.58%) | 0.621# |
| Heart Failure | 26 (44.07%) | 18 (15.79%) | 44 (25.43%) | <0.001# |
| Hypertension | 55 (93.22%) | 97 (85.09%) | 152 (87.86%) | 0.120# |
| Cardiac Insufficiency | 3 (5.08%) | 2 (1.75%) | 5 (2.89%) | 0.339* |
| Peripheral Vascular Disease | 2 (3.39%) | 5 (4.39%) | 7 (4.05%) | 0.999* |
| Diabetes | 26 (44.07%) | 49 (42.98%) | 75 (43.35%) | 0.999# |
| Chronic Kidney Disease | 23 (38.98%) | 23 (20.18%) | 46 (26.59%) | 0.008# |
| On Dialysis | 1 (1.69%) | 1 (0.88%) | 2 (1.16%) | 0.999* |
| COPD | 8 (13.56%) | 15 (13.16%) | 23 (13.29%) | 0.999# |
| Alcohol Abuse | 1 (1.69%) | 1 (0.88%) | 2 (1.16%) | 0.999* |
| Smoker | 34 (57.63%) | 60 (52.63%) | 94 (54.34%) | 0.532# |
‡: Kruskal-Wallis Test, *: Fisher’s Exact Test, #: Chi-Squared Test
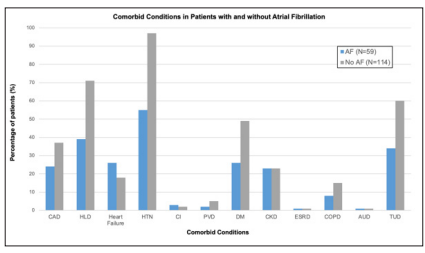
Figure 2: Comparing the Relative Frequency of Various Comorbid Conditions in Patients with LA Enlargement with and Without Associated Atrial Fibrillation. (CAD: Coronary Artery Disease; HLD: Hyperlipidemia; HTN: Hypertension; DM: Diabetes Mellitus; CKD: Chronic Kidney Disease, Stages II-IV; ESRD: end Stage Renal Disease; COPD: Chronic Obstructive Pulmonary Disease; AUD: Alcohol Use Disorder; TUD: Tobacco Use Disorder)
Our study shows that left atrial enlargement was the only echocardiographic finding with a statistically significant association with TIA in our study, with almost 40% of our sample population showing LA enlargement greater than 4 cm on TTE. This is consistent with findings of previous studies investigating the relationship between LA enlargement and CVA [6]. Unsurprisingly, approximately one third of those with LA enlargement carried a new or previous diagnosis of atrial fibrillation, which is also consistent with established relationship between AF and LA dilation [7]. However, there is little to suggest that the finding of LA enlargement has any bearing on management in the setting of TIA. This is suggested by the fact that less than 6% of patients with LA enlargement and no known diagnosis of AF underwent further evaluation via cardiac monitoring. Even though TTE facilitated the discovery of LA enlargement following TIA, a minority of patients underwent appropriate subsequent evaluation via cardiac monitoring to determine the underlying cause of LA enlargement.
Demographics of our population did show significant associations between TIA and various comorbid conditions. Specifically, conditions such as hypertension, dyslipidemia, smoking history, and diabetes mellitus were the most common comorbidities, and all had relatively high incidence in our study population. These are all known risk factors for TIA alongside increasing age, although age was difficult to assess in our population since all patients over the age of 89 are all considered 90 years of age to conform with HIPAA privacy protections in research [8].
Other limitations in our study are related to sample size and the ability to quantify information regarding management changes based on specific echocardiogram findings. Retrospective approaches have (by definition) limited control over the homogeneity of information recorded or available in the medical record. Low incidence of certain TTE findings in our sample population also limited our ability to perform statistical analysis, as was the case for mitral stenosis, PFO, atrial septal defect, thrombus, atrial myxoma and vegetations. Our study was also limited given the lack of follow-up period. More specifically, cardiac monitoring was limited to less than a 30-day period, and was performed at outpatient cardiology offices with results not included in the medical record. This combined with the relatively homogenous ethnic/racial demographics of our sample population (i.e., 84% Caucasian) limits the generalizability of our results. Ultimately a larger sample size and greater degree of followup would allow for further insight into the significance of TTE findings in workup of TIA.
However, our study did reveal that LA enlargement was statistically more likely in patients with AF and comorbid heart failure or chronic kidney disease, versus patients without AF. This might suggest that TTE may have varying degrees of utility in the workup of TIA depending on underlying comorbidities. Given stronger associations with AF in our study, it stands to reason that TTE may provide a greater degree of utility for patients with underlying heart failure or chronic kidney disease, especially if TTE improves rate of discovery of AF or other contributors to cardioembolic phenomena. Future prospective studies could further investigate the effect of increased utilization of cardiac monitoring on clinical outcomes in patients with underlying CKD or heart failure, especially if they are found to have LA enlargement in the workup of TIA.
This could include utilizing new technologies such as smart watches and other wearable devices to improve the implementation of cardiac monitoring following TIA. These devices have already demonstrated reliable diagnostic utility in the detection of atrial fibrillation [9]. The diagnostic superiority for implantable cardiac monitoring versus conventional follow-up for detecting atrial fibrillation in the setting of cryptogenic stroke has been well demonstrated in the literature [10]. However, the ever-increasing ubiquity and relatively low cost of wearable devices makes the argument for their investigation even more compelling, especially considering the non-invasive nature of monitoring and likelihood for improved patient compliance. Widespread availability of external wearable devices could theoretically improve the capacity for providers to implement cardiac monitoring for investigation of AF in the setting of TIA. Early evidence even suggests effectiveness in early detection of atrial fibrillation, which may have management implications regarding preemptive initiation of anticoagulation as a method of primary stroke prevention [11]. Certainly there is potential for medical application of these devices not just for the investigation of cryptogenic stroke or TIA (i.e., as a component of secondary prevention), which is compounded by an ever-expanding feature set and extensive voluntary adoption.
Obtaining echocardiograms in patients presenting with stroke-like symptoms and no findings on imaging is common practice. Based on our data, the only statistically significant echocardiogram finding seen in the general TIA population is left atrial enlargement. Left atrial enlargement is a well-known association of atrial fibrillation which could be a significant risk factor for future cerebrovascular accident (CVA) [6]. Of the patients with left atrial enlargement without atrial fibrillation (AF), only 6 of 114 (5.26%) were placed on monitoring on discharge. Based on the significant proportion of patients presenting with TIA who have concurrent left atrial enlargement and no atrial fibrillation, we anticipate that further prospective studies could provide greater insight into the role for cardiac monitoring in patients with LA enlargement in this at-risk population.
“This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.”
Author contributions: All authors contributed equally to this manuscript. N. Abdelsayed is the article guarantor.
Financial Disclosure: None to report.
