Author(s): Farzad Khajavi
Formulation of Ranolazine in the form of extended-release tablet in 500 mg dosage form was performed using Eudragit L100-55 as a retarding agent. Drug-release profiles were investigated in comparison with the reference Ranexa extended-release 500 mg tablet. F2 and f1 were calculated as 64.16 and 8.53, respectively. According to Peppas equation, the release of drug is controlled by diffusion (n=0.5). The tablets were put into accelerated stability condition (40?C, 75% humidity) for 3 and 6 months. The dissolution release profiles and other physical and chemical characteristics of the tablets confirmed the robustness and stability of formulation in this condition.
The goal of controlled delivery systems is to reduce the frequency and / or to increase the effectiveness of the drug by localization at the target site, thereby reducing the dose required to provide uniform drug delivery [1]. Drug release from the dosage form is controlled mainly by the properties of polymer and drug used in the preparations [2]. Cellulose ethers are found to accommodate a large percentage of drugs and are easy to use in tablets. They are also very stable over a wide range of conditions. In the presence of strong acid, water and heat a cellulose ether polymer will degrade by chain scission causing a loss of average molecular weight or viscosity. HPMC is often used to prepare matrix of sustain-release (SR) tablets because the polymer is non-toxic, easy to handle and do not require any special manufacturing technology [3-6] . It was evident that HPMC 2208 (methocel K4M premium) and carboxy vinyl polymers can release drugs for longer time by quickly forming a gel layer [1]. Eudragit® L100 is an anionic copolymer of methacrylic acid and methyl methacrylate with ratio of the free carboxyl groups to the ester groups of approximately 1:1. The polymer is insoluble in acid medium, hence when used in a coating layer of drug formulation, it will protect the acid-unstable-drug from degradation once it reaches the stomach [7-9]. In this work we formulated Ranolazine 500 mg extended-release tablet with the using of Eudragit L100-55 as a retarding agent. The stability results of tablets show that the formula is stable in this condition.
HCl, NaOH were purchased from Merck. Ranolazine from Aurobindo Pharmaceutical Co. Ltd., Microcrystalline Cellulose from Accent (India), Hydroxypropylmethyl Cellulose (HPMC 5 cps) from NIPPON SODA Co., LTD. (Japan), Eudragit L100-55 from Ashland (Belgium) and Magnesium stearate from Behansar Co. were provided. Ranexa Extended-release tablet 500 mg (Menarini Pharmaceutical Company (Italy)) were provided and used as reference tablets.
The ready to press powder of tablets was prepared by wet granulation method. The drug substance is kneaded with the internal phase (that contains Microcrystalline Cellulose, Eudragit L100-55, and Hydroxypropylmethyl Cellulose) after passing through 20 mesh screen for 15 minutes. This first mixture is kneaded with binder solution (prepared by dissolving of NaOH in purified water). The wet mass is dried in hot-air chamber at 50 °C, protected from light. The next step is passing the dried produced mass through oscillating granulator with 16 mesh screen. The dry granular mass is mixed with the Magnesium Stearate after passing through 80 mesh screen for about 5 minutes. After that, powder was compressed using a mini rotary press machine (Kambert Machinery Co. PVT. LTD. India) with 16 mm diameter oval concave punch.
The hardness of tablets was determined using hardness tester (Pharma Test PTB-311F, D-63512 Hain burg, Germany). The mean of the tablet hardness was calculated for ten tablets. The thickness of tablets was determined using a digital caliper (CE ISO 9001, Guanglu Electrical Co., LTD. China) and the results were expressed as mean values of ten determinations.
An in-vitro release of Ranolazine tablets was studied using USPdissolution apparatus II (Pharma Test, PTW S600, Germany). Nine hundred milliliters of HCl 0.1N, as the dissolution medium, was placed in the glass vessel, the apparatus assembled, and the dissolution medium equilibrated to 37 ± 0.5°C. The rotation speed of paddle was 50 rpm. At predetermined time intervals, the dissolution medium was removed for determining the drug concentration and fresh medium was replaced. The amount of drug released in the dissolution medium was measured using HPLC (Alliance, Waters e2695 series, USA) with C18 column (4.6 mmx25 cm, 5 μm particle size). The studies were carried out in six vessels. The cumulative percentage of drug release was calculated and plotted against time.
After press the tablets, the cores of tablets were obtained with the average weight of 664.2 mg, average thickness of 7.56 mm, average diameter of 7.48 mm and average hardness of 18.78 kp. The six of these tablets were selected for in vitro test study. Six tablets of Ranexa 500 were used as reference tablets. The dissolved amount of Ranolazine core tablet in dissolution medium was obtained at time intervals of 0.5 h, 2 h, 4 h, 8 h, 12 h and 24 h using an appropriate calibration curve for Ranolazine. Dissolution profiles of Ranolazine and Ranexa tablets were compared using a similarity factor equation (1) and difference factor equation (2).
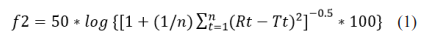
The similarity factor is a logarithmic reciprocal square root transformation of the sum of squared error and is a measurement of the similarity in the percent (%) of dissolution between the two curves where n is the number of time points, Rt is the dissolution value of the reference batch at time t, and Tt is the dissolution value of the test batch at time t. Two dissolution profiles are considered similar when the f2 value is ≥50.
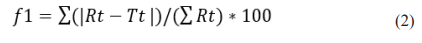
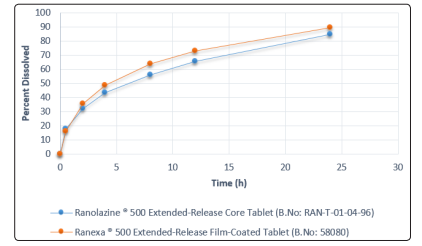
Difference factor (f1) should be equal or less than 15 between two similar dissolution profiles. f2 and f1 were obtained as 62.80 and 9.20, respectively, which indicate that the two tablets are similar. Dissolution profiles of two kinds of tablets are shown in Figure 1.
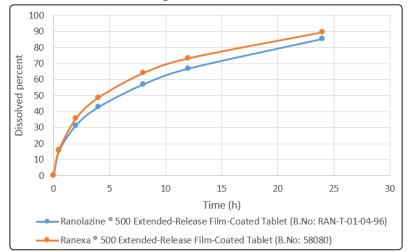
After coating the tablets with Opadry II85F24232 Pink, the six of these film-coated tablets were selected for in vitro study. f2 and f1 were obtained as 64.16 and 8.53, respectively, which indicate that the two tablets are similar. Dissolution profiles of two kinds of tablets are shown in Figure 2.
As expected drug release is decreased by increasing Eudragit L100-55 content in the formulation. Drug release of modifiedrelease Ranolazine 500 mg tablets obeyed from Higuchi equation Q=kHt 0.5 , where Q is the percent of drug released at time t and KH is a respective coefficient (R2 from plotting of dissolution (%) versus t0.5 was obtained as 0.9915 and 0.9877 for modified-release Ranolazine 500 mg core and film-coated tablet, respectively). From equation of Pepass (Q=k p t n ), n was obtained as 0.40 and 0.44 for modified-release Ranolazine 500 mg core and film-coated tablet, respectively. It shows that Fickian diffusion controls the drug release.
The purpose of this study is to provide evidence on how the quality of Ranolazine® 500 Prolonged-release Film-Coated Tablets change with time under the influence of environmental factors such as temperature and humidity. Stability testing will permit the establishment of recommended storage conditions, retest periods and shelf life.
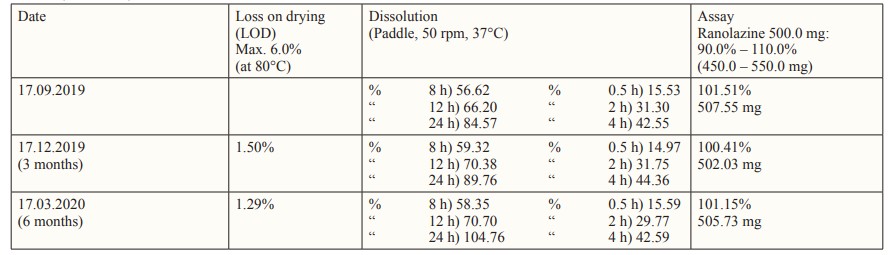
The trial batches were packaged in a cardboard box containing the appropriate number of PVC-Alu blisters. During accelerated stability testing following chemical and physical characteristics of samples are tested: appearance, average mass, thickness, diameter, disintegration time, loss on drying, dissolution and assay.
Accelerated stability results of Ranolazine® 500 Prolonged-release Film-Coated Tablets show that the tablets are chemically and physically stable. There is no change in assay of active substance, as well as any other tested parameters. On the basis of the 6 months accelerated stability testing, shelf life of 24 months is predicted at temperature below 30 °C and protect from moisture. The results are shown at Tables 1 and 2.
Table 1: Physical tests results of Ranolazine® 500 Prolonged-release Film-Coated Tablet Batch No.: RAN 500-T-01-4-96 (API Manufacturer: Aurobindo Pharma Limited) Accelerated condition: Temperature 40° ± 2°C, Relative humidity 75% ± 5% Packaging: Blister (PVC-Alu)
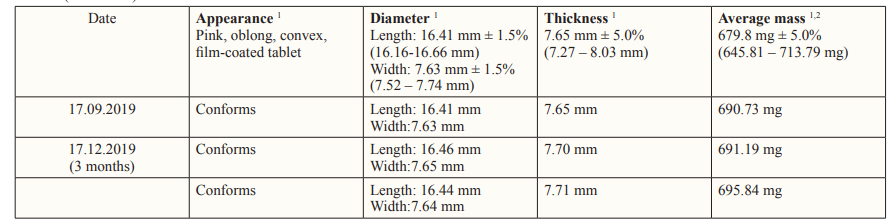
Table 2: Chemical tests results of Ranolazine® 500 Prolonged-release Film-Coated Tablet, Batch No.: RAN 500-T-01-4-96 (API Manufacturer: Aurobindo Pharma Limited) Accelerated condition: Temperature 40° ± 2°C, Relative humidity 75% ± 5% Packaging: Blister (PVC-Alu)
In this study formulation of Ranolazine Extended-release Tablet was performed using Eudragit L100-55 as a retarding agent. The dissolution profile was compared to the dissolution profile of reference product (Ranexa® 500) and accepted similarity was obtained. The accelerated stability studies were performed at 40°C and 75% humidity for 6 months and the results showed that the formula is stable in these conditions. Therefore, the formula is proposed for Ranolazine prolonged-release tablet.
