Author(s): Mario Vetri*, Alessia Cataldi, Adriano Naselli and Annalisa Vetri
Gender Identity Disorder (GID) is a condition characterized by a strong and persistent identification with the opposite sex. These people consider themselves victims of a sort of biological accident: “a soul in a wrong body”. There are numerous theories on the origin of transsexualism: genetic, hormonal and psychological causes have been hypothesized, but those currently most accredited are the neuroanatomical ones. The cornerstones of hormone conversion therapy (Gender Affirming Hormone Therapy, GAHT) are feminizing hormones for transgender women (MtFs or AMAB: Assigned Male at Birth) and virilizing for transgender males (FtMs or AFAB: Assigned Female at Birth). GID can be present among adolescents and older people. For adolescents is now accepted reversible treatment of puberty withdrawal with hormones that stops the progression of pubertal development in the biological direction not accepted; for elderly people are suggested GAHT in reduced doses. Physicians should consider and discuss with people with GID about fertility preservation, general and cancer risks. We present also data of 127 transsexual patients enrolled at the Garibaldi-Nesima Andrology Clinic in Catania (Italy) from 2003 to 2020. To optimize the conversion treatment with sex hormones, transsexuals require long-term follow-up. GAHT must be performed by a doctor who is familiar with these problems. Therefore, the “do-it-yourself” trend and the lack of medical and laboratory checks over time should be absolutely discouraged. Before proceeding with the surgical sex reassignment, it is recommended to refer to an endocrinologist and psychologist or psychiatrist for a period of 2-3 years. The transition surgical conclusion process must be practiced by a quality surgical team.
Gender identity - a person’s inner sense of feeling as male, female, or occasionally a category other than male or female - is not simply a psychosocial construct, but likely reflects a complex interplay of biological, environmental and cultural factors. Acceptance and recognition of individuals with gender identity disorders has increased during the last 20 years. GID can be of different types: male-to-female (MtF or AMAB) and female-to-male (FtM or AFAB). In most cases the first manifestations can occur even during childhood [1].
“Transgender” is an umbrella term commonly used to describe individuals whose gender identity and/or gender expression differs from assigned sex at birth. In the United States, approximately 150.000 youth and 1.4 million adults identify as transgender [2]. Individuals identifying as transgender often seek GAHT. However, hormone doses for transgender men and transgender women are not standardized; instead, they are subjectively personalized. Transgender people are a heterogeneous group of individuals who disagree with their biological sex and role assigned at birth. These individuals suffer extreme distress due to the discrepancy between their biological condition and their gender identity, and require psychological and medical intervention to correct primary and secondary sexual characteristics of their biological sex and to obtain those of the desired sex. For FtMs estrogen therapy in combination with antiandrogens is used, whereas those for FtMs testosterone are the main treatment. Estrogens and testosterone became commercially available for hormonal intervention starting from 1940. In 1967 Harry Benjamin published the book “The Transsexual Phenomenon” where he presented his experiences with hormone therapy to transgenders [3]. In 1979, however, that the first standards of care for transgenders were published by the Harry Benjamin International Gender Dysphoria Association, later renamed The World Professional Association of Transgender Health (WPATH) [4]. The last edition of the standards of care (Version 7) has been published in 2011 and version 8 will arrive in the next future. Ad-additional guidelines for hormonal treatment have been published by the Endocrine Society [5,6].
Studies about the real prevalence of GID are scarce and probably underestimated. It’s also true that the number of individuals who require endocrine treatment for their GID is increasing as we’ll demonstrate later. According to the reported statistics, the prevalence of transsexuality is 6 per 100.000 people worldwide, is more common among men, with reports of 1 per 30.000 (MtFs), and it is less common in women (FtMs, 1/100.000) [7,8].
To date, there’s no clear explanation for the etiology of transsexuality. In this regard, several factors like prenatal stress, parental sexual relations, genetic disorders, hormone structure, neuroanatomical problems, and some environmental factors, should be considered. Studying structure and function of males and females’ brains has shown some differences in the “hemispheric lateralization” (that is, the fact that the two hemi-spheres specialize in different functions), considered more distinct in males and minor in females. GID is still an entity with unknown causes [9,10]. There are many theories that have followed over the years and that have tried to find a scientific explanation for this condition.
The first theories on transsexualism date back to the first half of the twentieth century. Psychological theories attempt to explain transsexualism through various psychological factors, including altered family dynamics, traumatic experiences in childhood and alterations in normal sexuality. According to D. Cauldwell, transsexualism was nothing more than an exclusively psychological disorder, induced by various psychosocial factors. A desire to belong to the opposite sex appears completely normal in childhood for women, due to a sense of envy towards the penis for the great admiration one feels for one’s mother [11]. For Harry Benjamin the “traumas” suffered during childhood and adolescence or wrong situations can act on a (more or less labile) genetic and endocrine makeup in such a way to induce a disorder like transsexualism. The basis on which psychological theories are based is therefore the concept that gender identity is modifiable and depends, in part, on the genetic basis of the individual, in part on a large number of psychological variants that influence the subject during his sexual development. From the psychological perspective there are also other theories. According to the “non conflictual hypothesis” the individual with GID has had a female gender nucleus since early childhood. This “femininity” has been adopted in a nonconfrontational way by the entire family system, where more often it is the mother who is gratified by this inverted gender identity. The “conflictual hypothesis” believes that the request for sex change is the result of a “pathological compromise”. In this context, transsexualism is considered a defense against homosexuality seen as a form of perversion, narcissistic disorder or perturbation of the separation-identification phase [12].
In transsexuals, sexual differentiation of the brain and genitals may go into opposite directions suggesting a neurobiological basis of GID. One classic way to test whether a trait is influenced by genetics is studying identical twins; they have the exact same genetic background, and are usually raised in the same environment [13]. Non-identical twins, however, share only half their genes, but tend to also be raised in the same environment. Thus, if identical twins tend to share a trait more than fraternal twins, that trait is probably influenced by genetics [14]. Several studies have shown that identical twins are more often both transgender than fraternal twins, indicating that there is indeed a genetic influence for this identity. The first studies showing a genetic correlation with GID date back to the 1990s by the group of Bailey and Bell, based on the study of monozygotic and dizygotic twin brothers; but only in recent years, research lines have focused on studying the genes that control human sexuality [15]. In 2009 an Australian team focuses on the evaluation of androgen receptor polymorphisms, estrogen receptor and aromatase genes in transsexual MtF patients versus heterosexual men controls [16]. This study demonstrates a statistically significant association in CAG triplet polymorphism and androgen receptor gene, where transsexuals have longer polymorphisms than controls; while for the remaining two genes there are no relevant associations. More expanded CAG triplets in the androgen receptor gene reduce the link between this protein and the co-activating proteins, thus reducing the action of testosterone, which is essential in the embryological development phase to induce complete masculinization of the brain. Reduction of testosterone stimulation during intra-uterine brain development, inducing a cerebral feminization, develops a female gender identity in such patients. In FtM transsexuals has been studied the “single nucleotide polymorphism” for the CYP17 gene, which produces an enzyme to creates dehydroepiandrosterone and androstenedione, in turn peripherally metabolized into testosterone and estradiol. Mutant variant of the CYP17 gene in FtM transsexuals, determining an increase in serum values of testosterone and estradiol and inducing an alteration in the normal brain development of sexuality, leads to identification in the male gender.
An aberration in the early sexual differentiation of various brain structures has been suggested. Animal experiments have revealed that sexual differentiation of the brain is mainly due to an influence of testosterone, which acts both through the androgen receptors and through the estrogen receptors after its conversion (aromatization) into estradiol. In human model has been postulated that, for instance, FtMs may have been exposed to inadequate levels of estrogen during development (Figure 1) [17]. This phenomenon could have two causes: 1) not enough estrogen in the fetus’s immediate environment, or 2) enough estrogen in the environment, but poor sensitivity in the fetus.
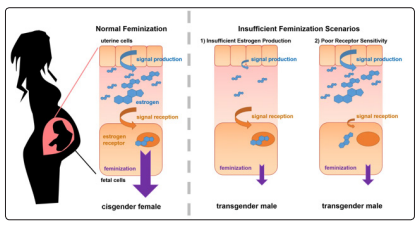
Figure 1: FtMs: Insufficient Feminization
Logistic regression analysis of gene-gene interaction for susceptibility to transsexualism in sex hormone related genes has not proven that genetic variants of genes associated with sex hormones confer individual susceptibility for MtF or FtM transsexualism [18]. There are few reports describing chromosomal abnormalities in transsexuals. In rare cases, transsexualism and sexual chromosomal multiplicity coexist. Six cases of MtF transsexuals with 47, XYY chromosomal pattern have been previously reported. Has been published a case of FtM transsexual with 47, XXX karyotype, suggesting that sexual chromosomal abnormalities in some transsexuals may cause a vulnerability to development of a gender identity disorder [19].
Several studies have identified how the sexual diversity between men and women does not exclusively involve the genitals, but also the development of different brain areas [20]. Among these areas, belonging to the limbic system (including hypothalamus and amygdala), the most relevant has been identified in the nucleus of the terminal stria (BSTc) located in its central portion as a “highly dimorphic area”. This area, being part of the hypothalamic structures, has been extensively studied initially in rodents and subsequently in humans, and it’s involved in sexual behaviors [21]. The volume of this area appears to be influenced by the stimulation of sex hormones during brain development. The BSTc volume is normally greater in men than in women and MtF transsexual patients have a volume comparable to that of heterosexual women (Figure 2). In this matter gender identity develops from the complex interactions between sex hormones and brain during its development; moreover, this evolution appears to be genetically predetermined and is not influenced by hormonal stimuli during the adult phase [22].
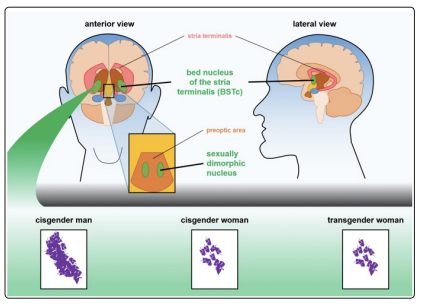
Figure 2: Transgender women (MtFs) tend to have brain structures that resemble cisgender women, rather than cisgender men. Two sexually dimorphic areas of the brain are often compared between men and women. The bed nucleus of the stria terminalis (BSTc) and sexually dimorphic nucleus of MtFs are more similar to those of cisgender woman than to those of cisgender men
Studying somatostatin neuron numbers (physiologically expressed in this area) present in the BSTc, as a marker, it has been demonstrated a greater number of somatostatin positive neurons in BSTc in men than in women (almost double) and that, in MtF transsexual patients, the number of these neurons was in a typically female range; hormonal variations during adulthood seems not influence the dimorphism of this area (Figure 3) [23].
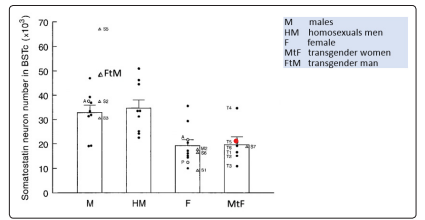
Figure 3: Somatostatin neuron numbers in the bed nucleus of BSTC
In 2000s has been discovered another hypothalamic area involved in the determination of gender identity and characterized by an important sexual dimorphism; this area, the hypothalamic uncinate nucleus, is composed by 2 sub-nuclei, called interstitial nuclei of the anterior hypothalamus (INAH) 3 and 4. INAH3 nucleus (Figure 4) was 2 times larger in males than in women [24].
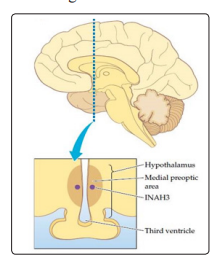
Figure 4: The nucleus of cells within the third interstitial nuclei of the anterior hypothalamus (INAH-3) markedly differs in size between males and females. It was found that the nucleus in males is an average of two to three times larger than it is in females.
In MtF transsexuals the volume and number of these neurons was comparable to that of the cisfemale controls while in FtMs superimposable to the cismale controls. In addition, studying women in both pre and postmenopausal phase, was possible to demonstrate that estrogen did not affect INAH3 and that there was no role in cerebral feminization by estrogen on the brain (Figure 5) [25].

Figure 5: M: males F: females MtF: transgender women CAS:
castrated males
Pre-men: premenopausal women Post-men: postmenopausal
women
“Gender Nonconformity” means the level to which an individual’s gender identity differs from the common cultural norms for a person of a particular sex. By GID we mean the malaise or stress caused in a person who feels his own gender identity other than the sex assigned to him/her at birth. Only a few of “nonconforming people” have GID in their life. Treatments are available to help people with such distress to explore their gender identity and find a gender role in which they feel comfortable. The WPATH recommends that GATH should be initiated once the psychosocial assessment has been completed, the patient has been considered a candidate for therapy and informed consent (indication of risks and benefits) is obtained. The clinician should evaluate the magnitude, duration, and stability of any gender dysphoria or incongruence. It is recommended that before staring GAHT, patients should have had documented “real-life experience” of several months prior to the hormone administration or a psychotherapy (no less than three months) after the first evaluation. Criteria to start GAHT include: persistent well-documented gender dysphoria diagnosed by a mental health professional experienced in the field; ability to make a fully informed decision and to give consent for treatment; the age of majority; finally, significant medical and/or mental comorbidities were excluded. The goal of hormone therapy for transgender people it’s more closely aligns an individual’s physical appearance with their gender identity. The purposes are: obtain the maximum of feminization or virilization in the shortest possible time; incur as few physical and emotional side effects as possible. Feminizing therapy is taken to produce female secondary sex characteristics and suppress or minimize male characteristics; the goal of masculinizing therapy is the opposite. Feminizing and masculinizing hormone therapies are partially irreversible treatments. Not all gen-der-diverse persons require or seek hormone treatment; however, those who receive treatment generally report improved quality of life, self-esteem, and anxiety.
An increasing number of preadolescents and adolescents, identifying as “transgender”, are seeking medical services to enable the development of physical characteristics consistent with their affirmed gender. The new guidelines of the Endocrine Society have also considered the treatment of dysphoric adolescents by suggesting to interrupt the pubertal maturation once they have entered the Tanner G2/B2 stage by suppression with GnRH agonists (Figure 6) [26].
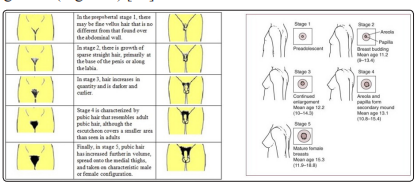
Figure 6: Tanner stage ≥G2/B2
As early as 2011, the Dutch experience indicated that adolescents who met the diagnostic criteria for gender inconsistency, and were eligible for treatment, could be subjected to therapy to block pubertal development [27,28]. After the pubertal block, it is recommended to start treatment with hormones consistent with the desired sex at gradually increasing doses from the age of 16. Refusing timely medical interventions for adolescents can prolong gender dysphoria and contribute to a physical appearance that could lead to abuse and stigmatization [29]. Since the level of gender-related abuse is strongly associated with the degree of psychiatric distress during adolescence, denying the suppression of puberty and subsequent feminizing or virilizing hormone therapy is not a “neutral” option for adolescents. Furthermore, onset of puberty in transgender youth is often ac-companied by increased “gender dysphoria”: clinically significant distress related to the incongruence between one’s affirmed gender and one’s “assigned (or natal) gender”. Transgender youth and adolescents are at significantly increased risk for life-threatening behaviors [30]. Interviews of transgender youth in New York City (55 MtFs, 24 FtMs) demonstrated that 45% had experienced suicidal ideation, whereas 26% had attempted suicide [31]. After endogenous puberty has been blocked, let’s use cross-sex hormones. For hormonal interventions in transgender adolescents see Table 1.
a. Medroxyprogesterone acetate orally (up to 40 mg/d) or im
(150 mg every 3 mo): inhibition of hypothalamic-pituitary
gonadal axis and direct inhibition of gonadal steroidogenesis
(FtM and MtF);
b. Spironolactone (25 to 50 mg/d with gradual increase to 100-
300 mg/d orally, divided into twice daily dosing): inhibition
of T synthesis/action (MtF);
c. Cyproterone acetate (gradual increase up to 100 mg/d orally;
not available in United States): inhibition of T synthesis/
action (MtF);
d. Finasteride (2.5-5 mg/d orally): inhibition of 5-α-reductase,
blocking conversion of T to DHT (MtF).
a. Transdermal: twice weekly patches (6.25 g [achieved by
cutting a 25-g patch] with gradual increase to full adult dose);
b. Oral/sublingual: daily (0.25 mg with gradual increase to
full adult dose of 6-8 mg/d);
c. Parenteral im (synthetic esters of 17-estradiol): estradiol
valerate (5-20 mg up to 30-40 mg/2 wk) or estradiol cypionate
(2-10 mg/wk).
a. Parenteral im or sc (synthetic esters of T): T cypionate or
enanthate (12.5 mg/wk or 25 mg/2 wk, with gradual increase
to 50-100 mg/wk or 100-200 mg/2 wk);
b. Transdermal (consider once the full adult T dose has been
achieved parenterally): patch (2.5-7.5 mg/d) or 1% gel (2.5-
10 g/d of gel 25-100 mg/d of T).
There is no “ideal” GAHT. The aims are to obtain the maximum of feminization or virilization in the shortest possible time and to incur the least possible number of physical and emotional side effects. Candidates for hormone therapy should be 18 years old and able to make and give informed consent for therapy. Hormone treatment is expected to be life changing and will result in some irreversible effects; potentially serious complications of hormone therapy may occur. Because the use of medications for cross sex hormone therapy is considered to be “off-label”, all patients will be asked to provide signed consent for hormone therapy. Patients must consent to therapy after being informed of the potentially irreversible changes in physical appearance, fertility potential, and social circumstances, as well as other potential benefits and risks [32]. Estrogens are the primary class of medications used for feminizing therapy and include oral, parental, and trans-dermal methods of delivery. Antiandrogens, such as spironolactone, cyproterone acetate, or 5α-reductase inhibitors, may also be used, often as adjunctive medication, to suppress testosterone. Testosterone is the mainstay of masculinizing therapy and is available in oral, buccal, injectable (subcutaneous or intramuscular), implantable pellet, or trans-dermal forms.
For MtFs using estrogens, high to moderate risk adverse outcomes include thrombo-embolic disease, hyperprolactinemia, breast cancer, cholelithiasis, and hypertriglyceridemia. Given these risks,
it is strongly recommended to encourage tobacco cessation, although smoking is not an absolute contraindication to estrogens. If a MtF has a prior history of a sex hormone-responsive cancer (breast or pituitary), consultation with an oncologist before initiating therapy is critical. All patients who will initiate hormone therapy must have a complete psycho-social history and physical exam along with screening lab tests.
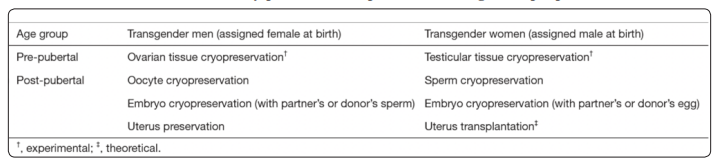
Most transsexual men and women are of reproductive age at the time of transition and have post-transition relationships. These people should consider fertility issues before initiating GAHT. There are strong arguments for counseling transgender patients about fertility preservation. Reproductive desire could be high among transgenders, but re-productive options are surprisingly low [33]. Clinical options for fertility preservation in transgenders are determined by pubertal status and stage of medical or surgical transition. In MtFs, cryopreservation of sperm is recommended before starting hormone therapy. In FtMs there are more options for preserving fertility: preservation of oocytes, ovarian tissue, embryos or even uterus (Table 2).
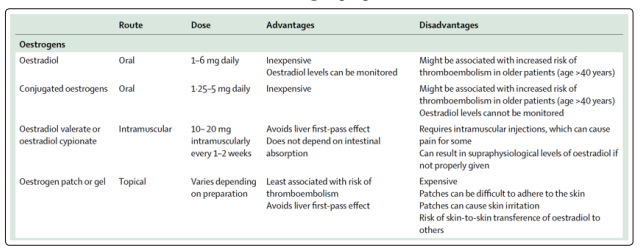
The optimal medical treatment offered to MtFs includes estrogens plus an androgen blocker (the latter can be stopped after gonadectomy) to reduce endogenous testosterone levels from the male range (10.4-34.7 nmol/L [300-1000 ng/dL]) to the female range (<1.7 nmol/L [<50 ng/dL]) [34,35]. Mainly 17-estradiol is used can be administered by oral, transdermal, or parenteral routes. Actually, ethinyl estradiol and conjugated estrogens are no longer recommended due to the high risks of venous thromboembolism. The usual dosing of oral estradiol is 2 mg daily, which can be titrated up to 6 mg if tolerated. If using injectable estradiol this is given biweekly. Transdermal estradiol patches are associated with lower risk of thromboembolism and may be preferred when age is greater than 45 years and there is history of venous thromboembolic disease, cardiovascular disease risk factors, or tobacco use. Orchiectomy is the most effective means of decreasing testosterone levels, but many transgender women choose medical treatment only, particularly early in their presentation. Thus, many have intact testes and may require relatively high estrogen doses to suppress testosterone into the female range, even with an adjunct anti-androgen agent. The usual approach is to start the estrogen and antiandrogen therapies concurrently (Table 3).
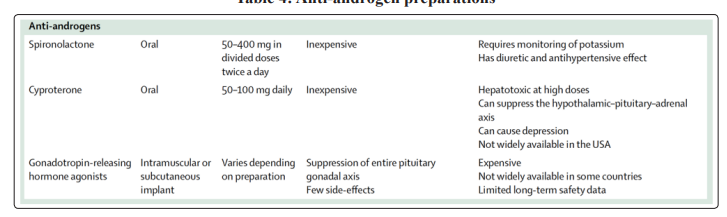
Chronic antiandrogen therapy is typically discontinued after orchiectomy. The androgen blocker used most frequently in the United States is spironolactone, an oral mineralo-corticoid-receptor antagonist with antiandrogen properties. Spironolactone, a potassiumsparing diuretic and antiandrogen medication, is a drug primarily used for patients with heart failure or liver disease, and may also be used for chronic antiandrogen therapy. However, dosing of spironolactone in GAHT may be 2- to 4-fold higher than for patients with heart failure or liver disease, with regimens in a dosage range of 100 to 400 mg daily (not 25 to 100 mg daily). Spironolactone given at gender-affirming dosages may increase potassium levels, reduce volume status or exacerbate acute kidney injury in the setting of an illness; however, the effects of such dosages have not been formally studied to date. Active monitoring of potassium and renal function in the acute setting and dosage adjustments, when needed, are important. If spironolactone therapy is discontinued, the dosage does not require tapering. Cyproterone acetate (CPA) is a synthetic progestogen with potent antiandrogen activity. Although not approved by the US Food and Drug Administration, it is approved in other nations (Europe, Canada). Some transgender individuals use this synthetic progestogen. Although often well tolerated and effective, the use of CPA has been associated with a risk of deep vein and arterial thrombosis, as well as rare cases of fulminant hepatotoxicity. Recently the use of CPA has been associated with the onset of meningiomas in the general and transgender populations. The higher risk seems to be related to the dose used and therapy duration;the incidence of meningiomas seems to be higher in MtFs than in the general population [36]. Gonadotropin-releasing hormone (GnRH) analogues (most frequently used in Europe), such as leuprolide acetate, are neuro-hormones that block the GnRH receptor, decreasing the release of luteinizing hormone and follicle-stimulating hormone. Such agents may be used to suppress puberty in pediatric or adolescent transgender patients or for suppression of menstruation in FtMs. There are limited data on prolonged use of GnRH analogues in adults, although deleterious effects on bone mineral density may be seen (Table 4).
Finasteride or dutasteride (5α-reductase inhibitors), are used for transgender women unable to tolerate spironolactone, those who seek partial feminization, or those who continue to exhibit virilized features after complete androgen blockade or orchiectomy. These medications may also be prescribed for transgender men experiencing androgenic hair loss as a result of testosterone therapy. There are no additional considerations in using these agents other than what is known for cisgender men, where it can lead to dizziness or orthostatic hypotension, although targeted studies in transgender patients have not been performed to date. Feminizing hormonal regimens in MtFs result in several body changes that sometimes are not reversible (do not change the voice). These regimens, especially CPA, will usually decrease libido and cause erectile dysfunction and poor ejaculation (Figure 7).
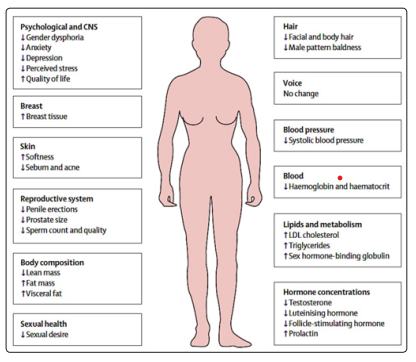
Figure 7: Transwoman (MtF)
Testosterone treatment is the principal hormone of GAHT for FtMs and nonbinary identified people [37]. Testosterone can be administered by injection, transdermal (patch or gel), oral formulation and, recently, nasal routes (not really recommend in Trans people). There are no studies to select a particular route, so this decision is made with the patient, considering issues such as cost, comfort with self-injection, possibility of testosterone gel transferring to others, and understanding that low-dose gel may not adequately suppress menses. The usual dose of testosterone cypionate is 50 to 100 mg subcutaneous injection (or 100-200 mg intramuscularly every 2 weeks). For patients interested in modified regimens, possibly because they have a nonbinary identity, maximum dosing is not required (Figure 8).
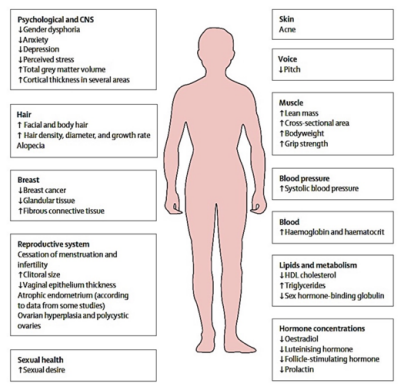
Figure 8: Transman (FtM)
Testosterone effects usually modify fat distribution (more fat in the abdominal area), and induces other changes (Figure 8), but even if frequently causes amenorrhea, it is not an effective contraception method.
Some transgender people start the transition to their experienced gender at an older age, even past the age of 50 or 60 years. There is very little literature on ageing transgender persons. The medical care of the elderly transgender patient is largely identical to that of the elderly non-transgender patient [38]. There’s no evidence that effect manifestations of GAHT will be less in the elderly than in younger people. Patients who have been prescribed oral conjugated estrogens should be switched to transdermal or intramuscular administration of hormones. FtMs who have been receiving testosterone, particularly if they have undergone hysterectomy and oophorectomy, will require exogenous androgens for life.
For elderly FtMs commencing hormone therapy, testosterone, administered Trans dermally or by intramuscular injection, can attenuate dysphoria and depression. Age itself should not be regarded as a contraindication to start with hormonal therapy, but the risks of side-effects may be higher at an older age.
Today, also the diagnostic criteria from DSM-5 and ICD-11 reflect a broader understanding of a new class of trans-individuals. It encompasses diverse identities and treatment requests, including trans individuals not wanting or having decided against GAHT. According to recent data trans-individuals not seeking GAHT are significantly older; such people say to refuse GAHT to avoid transition-related suffering and because they don’t feel the necessity for treatment [39]. In older MtFs who wants full feminization and preservation, at the same time, of erectile function it’s possible to use only estrogens treatment or estrogens combined with low dosages of cyproterone acetate or 5-reductase inhibitors. In FtMs requesting partial masculinization, it may be possible to reduce the dose of testosterone or substitute it with nandrolone, an anabolic steroid ad-ministered via intramuscular injection. So, we must accept alternative hormonal treatment regimens, other than those reported in current guidelines, for such non-binary transgender individuals, to try to improve their psychological well-being and quality of life [40].
The effects and timing of hormone therapy for transsexuals are subjective and depend on multiple factors, including the number and response of estrogen and androgen receptors naturally present in the patient’s tissues and the time of life relative to the individual’s development and when therapy started. Some effects will be visible after a few months, others will take more than 12- 24 months to appear. The aesthetic result sought by patients is also subjective, which does not necessarily have to be extremely feminine or masculine, but could also be, for instance in MtFs, an androgynous result [41].
Monitoring feminizing therapy for MtFs must be done every 2-3 months for the first year and then 1-2 times a year [42]. Levels of testosterone, estradiol, prolactin and electrolytes (the latter for those who use spironolactone) should be checked. Routine screening checks (breast, colon, prostate) should still be performed. Screening for osteoporosis should be done in individuals at risk (previous fractures or prolonged periods of hypogonadism) and after the age of 60 (Table 5).
Monitoring virilizing therapy for FtMs must be done every 2-3 months for the first year and then 1-2 times a year. Testosterone levels, liver function indices and cholesterol should be checked. Screening for osteoporosis follows the indications of MtFs; but in FtMs, if cervical or breast tissue is still present, the required tests (PAP test, mammography) must be performed. (Table 6)
Note: during the first 3-9 months of testosterone treatment, total testosterone levels may be high due to high sex hormone binding globulin levels in some biological women.
Generally, there are no absolute contraindications to hormonal conversion therapy for transgender people [43]. For MtFs are: severe arterial hypertension, thromboembolic and/or cerebrovascular disease or severe liver disease [44]. If we consider the relative contraindications, they are massive cigarette consumption, family history of breast cancer, high prolactin or severe obesity for MtFs; diabetes mellitus, dyslipidemia and obesity for FtMs. There are risks associated with GAHT, depending on whether you are considering feminizing hormones or those with virilizing action. They range from a probable increase in venous thromboembolic disease to a possible increase in non-insulin-dependent diabetes mellitus. Several cases of prolactinoma have been reported in MtFs in GAHT with estrogens and CPA, and this also occurred in subjects with normal prolactin concentrations even before therapy [45,46]. Although the causal link has not been definitely established, it is advisable that serum prolactin levelsare continuously monitored in MtFs under estrogenic + CPA treatment (Table 7) [47].
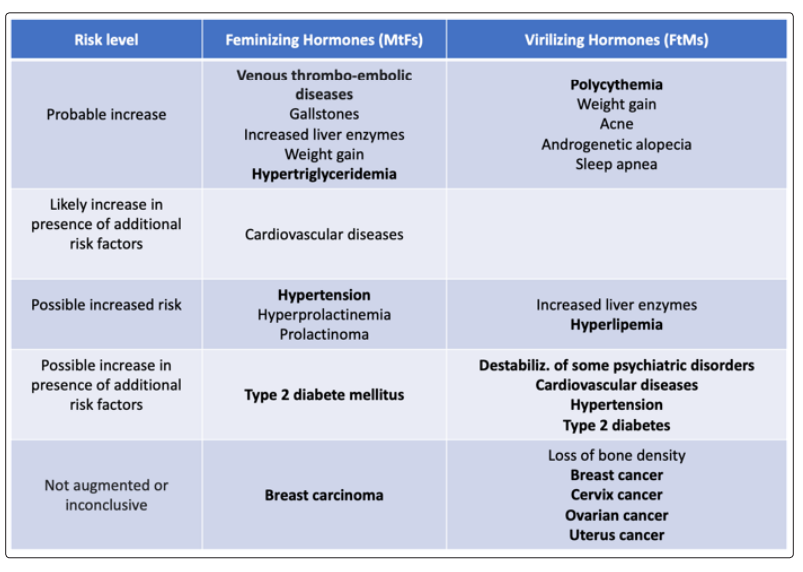
Much has been discussed about the possible development of GAHT-related tumors [48]. There are currently no established cancer screening guidelines, recommendations, or protocols for transgender patients at any point in their transition. However, since the first documented hormone treatments of transsexuals date back to 1970 and the time of exposure to hormones may have been too short for tumors to appear, we are not yet able to express an opinion of certainty in this regard. We also remember that transsexualism is a rare phenomenon and that, until now, there are no cancer registries in transsexuals using GAHT.
Estrogen in combination with antiandrogen therapy in MtFs can stimulate breast lobules, ducts, and acini. While for a long time it was believed that the risk of breast cancer in MtFs receiving hormonal therapy was not higher than those of men, most recent evidence shows that transwomen receiving hormonal therapy do have a 46-fold higher risk for breast cancer compared to men [49,50]. Addition of progestin to estrogen can leads to an increasing risk of breast cancer in ciswomen [51]. Although evidence regarding breast cancer and the usage of the progestogenic CPA is lacking, some data suggest that CPA should be continued no longer than necessary.
Prostate cancer is very rare among transwomen [52]. Based on available evidence it does not seem necessary to screen transwomen in a different way to cismen, for which population-based screening is not recommended. But similarly, a transwoman with a firstdegree male relative with prostate cancer should be made aware of her increased risk and prostate cancer antigen (PSA) testing should be discussed to allow informed decision making. However, when interpreting PSA values in this context, it has to be kept in mind that suppression of testosterone by antiandrogens or due to gonadectomy lowers PSA values.
Serum prolactin concentrations usually rise slightly in response to estrogen administration and more so by CPA. Based on case reports, it was initially believed that prolactin concentrations in MtFs had to be regularly monitored because of their increased risk for prolactinomas. Surprisingly, a very recent cohort study suggests that the occurrence of prolactinomas in transwomen using hormonal therapy is not higher than that in cis-women, and that regular prolactin checks are not necessary [53]. However, CPA should be continued no longer than necessary.
Several meningiomas have been reported in transwomen. The current estimated incidence rate of this type of tumor is 33/100.000 person-years. This incidence rate is 4 times higher than the incidence rate in ciswomen and 12 times higher than the incidence rate in cismen. It has been suggested that the occurrence of meningiomas in transwomen is mainly related to CPA usage as progesterone receptors are abundantly expressed in human meningiomas [54]. Since the occurrence of meningiomas is still rare in trans-women, regular screening for this type of tumor seems not necessary. It is recommended to continue CPA no longer than necessary.
As sexually transmitted infections may be more prevalent in MtFs, tumors related to sexually transmitted infections, such as Kaposi sarcoma or anal cancer, may also occur more often. Indeed, high incidences of these types of tumors have been found in the transgender population. Some case reports have been published on cancer in surgically constructed organs like the neo-vagina in transwomen. While the incidence of these types of tumors seem to be very low it is important to be aware of this possibility
Cases of breast cancer have been reported in FtMs before mastectomy. It is important to know that in MtFs not all glandular tissue is removed during a mastectomy. Indeed, several cases of breast cancer have been reported in transmen after mastectomy [55]. The incidence of breast cancer in FtMs who have received mastectomy seems higher than in cismen, but much lower than in ciswomen. While physicians and FtMs have to be aware of their risk of breast carcinoma after mastectomy, it seems unnecessary for transmen to participate in the screening programs for women. However, for MtFs with a breast cancer genetic predisposition one could be considered some radical forms of mastectomy.
Not all FtMs choose to remove their uterus. Menstruation usually ceases in transmen receiving testosterone therapy. Testosterone can be converted into estradiol, which may induce proliferation of the endometrium. These mechanisms may induce a higher risk of endometrial cancer in FtMs [56]. It is also possible that the risk for endometrial cancer in FtMs using testosterone is lower due to complete atrophy of the endometrium. There is currently only 1 case of endometrial cancer reported in a FtM using testosterone. But it is important to know that, until recently, many countries required removal of female sex organs before FtMs could change their sex on the birth certificate. Therefore, long-term followup data about testosterone receiving transmen with a uterus are lacking. In transmen with non-cyclic vaginal blood loss, it’s recommended to perform a vaginal ultrasound.
FtMs, in whom the uterus has not been removed, have a risk of cervical carcinoma. Human papilloma virus is the most important risk factor for developing cervix carcinoma. To date, only 2 cases of cervical carcinoma in transmen have been described [57].
Endometrial epidermal growth factor receptor, which is stimulated by testosterone, is commonly found in ovarian cancer cells, and its expression has been associated with poor prognosis. However, whether testosterone therapy increases the risk for ovarian cancer in FtMs has not been elucidated yet. To date, few cases of ovarian cancer have been reported in FtMs using testosterone [58,59].
Cases of breast cancer (MtFs + FtMs), benign prostatic hyperplasia and prostate cancer (MtFs), ovarian or endometrial cancer (FtMs), as well as cancers of non-reproductive organs (MtFs + FtMs) are extremely rare. Mortality in transsexual population is mainly related to increased suicide risk, HIV infection or drug abuse. Therefore, regular medical follow-up is always recommended.
At the Garibaldi-Nesima Andrology Clinic in Catania (Italy) a total of 127 trans-sexual patients were enrolled from 2003 to 2020. Among them we’ve been select 42 FtMs and 52 MtFs to retrospectively analyzed baseline and post-therapy hormonal and phenotypic characteristics. During the years and especially during the last 5 years, the number of patients who required hormone conversion therapy has been increasing both between MtFs and FtMs (Figure 9).
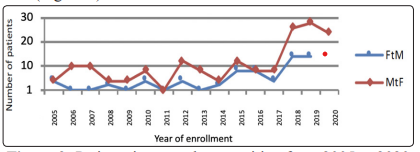
Figure 9: Patients interested to transition from 2005 to 2020
Patients who came to our observation in recent years have started GAHT earlier than patients who came to our Center in the early 2000s (Figure 10).
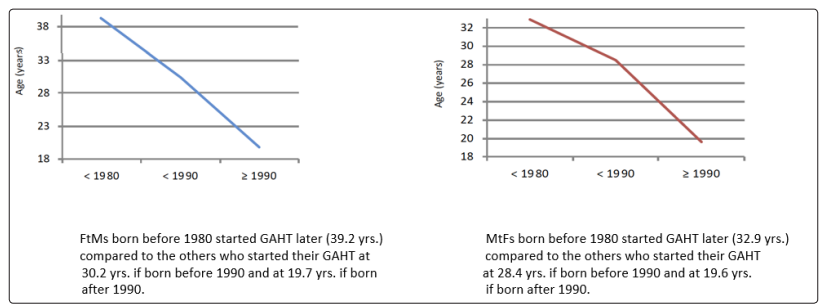
Figure 10: Timing of starting GAHT for FtMs and MtFs
FtMs were treated with intramuscular TE in different preparations. We therefore retrospectively analyzed Body Mass Index (BMI), haemochrome and hormonal (LH, FSH, Prolactin) measurements comparing baseline levels to post-therapy. We also compared TE formulations with each other. A total of 12/42 patients (Pts) reported pre-therapy anamnestic and clinical features suggestive of hyperandrogenism, while 18/42 showed adrenal androgen levels above the upper reference range (TE, δ4A, DHEAs). 31/42 Pts were treated with i.m. TE enanthate (TEe) and 11/42 with i.m. TE undecanoate (TEu) at different dosages. Pts with a baseline BMI <30 kg/m2 showed a mean BMI increase of 0,6-1,2 kg/m2 after starting TE administration; we observed maximum BMI increase after 12-18 months of treatment. 4 Pts with a pre-therapy BMI >30 kg/m2 showed a BMI decrease of -7,9 and -1,1 kg/m2 after 24 and 18 months of treatment respectively. Hematocrit (Hct), Hemoglobin (Hb), and Red Blood Cells count showed a similar increasing trend, with maximum values after 9-18 months of treatment; Hct reached values >50% (maximum 54,6%) in a total of 14 Pts, 4 of which treated with TEu; in those Pts low-dose acetylsalicylic acid orally was added to hormonal therapy. Mean serum PRL decreased throughout treatment from 20,1 to 9 ng/ml; minimum values were observed after at least 12 months of therapy. Mean LH and FSH levels didn’t show a regular trend throughout time and no correlation was found between gonadotropins and other follow-up parameters. Each patient required, as predictable, a different and tailored TE dose. TEe dosage ranged from 80 mg/21 days to 250 mg/15 days, whereas TEu ranged from 1000 mg/12 weeks to 1000 mg/21 weeks. TEu therapy provided more stable levels of testosterone, whereas spikes and drops in testosterone levels were observed during TEe treatment. The substantial price difference between these two drugs, however, prevented a higher rate of prescription of TEu among FtMs. During the initial followup 13/42 Pts (32%) complained of persistent uterine bleeding; 9 of them were taking TEe, 4 TEu. In 8 Pts bleedings easily disappeared after increasing the testosterone dose. These data, although limited by its retrospective nature, confirms in FtMs the higher prevalence of hyperandrogenism and the PRL decreasing trend throughout hormonal treatment. None of our Pts developed hypertension. No cases of ovarian pathology were detected; but it might be considered that bilateral ovariectomy could prevent the development of ovarian malignancies. The BMI increase of 0,6-1.2 kg/m2 after treatment might be due to fluid retention, or an in-crease in lean body mass or fat mass. This should be evaluated by body composition measurements in further studies.
MtFs hormonal management has been done using Estradiol Valerate (EV) and Cyprot-erone Acetate (CPA) at different dosages. We therefore retrospectively analyzed baseline and post-GAHT: serum testosterone (TE), LH, FSH and prolactin (PRL) measurements. 22/52 MtFs were treated with the same dose from the beginning, while 30/52 needed to increase the dose at least one time. A total of 4 patients were treated with EV 2 mg/die plus CPA 50 mg/die (2+50 mg), 24 with 4+50 mg, 12 with 6+50 mg, 4 with 8+50 mg, 3 with 8+100 mg and 5 with 4+25 mg. Patients usually started hormonal treatment with 6+50 mg, while further adjustments were made on the basis of clinical and laboratory findings. TE, LH and FSH suppression was obtained from the 3th month of treatment and it was maintained in a satisfactory way during the follow-up. Mean serum PRL increased from 8 ng/ml (baseline) to 30-35 ng/ ml (follow-up); during the follow-up 12 patients showed a PRL > 30 ng/ml, but no other therapy (cabergoline) was added. When adequately tailored for each patient according to clinical and previous laboratory results, each dose showed itself to be able to suppress hypothalamic-pituitary-gonadal axis without major adverse events. Lowest TE, LH and FSH levels were observed, as expected, with 8 EV+100 CPA mg
In our experience TE administration in FtMs appears to be effective in maintaining testosterone levels within physiological limits, well tolerated and safe with no apparent differences between the testosterone formulations used. Hormonal therapy for MtFs with EV plus CPA at different dosage was generally quite effective with almost none adverse events, even if the ability to induce full feminization can be variable depending on the Pts genetic background.
Not so many transgender people choose to have surgery in addition to hormonal therapy, even if long-term post-operative care and follow-up after surgical treatments for gender dysphoria are associated with good surgical and psychosocial outcomes. A variety of surgical approaches are available and should be tailored to the trans patient [60]. Mainly it deals with vaginoplasty in MtFs and neophallus in FtMs. While some types of surgery affect fertility, (vaginoplasty in MtFs or oophorectomy/hysterectomy in FtMs), others do not, such as breast surgery in both FtMs and MtFs. For GAS it is essential that anesthesia providers develop the knowledge and skills necessary for safely managing transgender patients in the perioperative setting. Some surgeons advocate discontinuance of hormones 2-4 weeks before surgery. Anesthesia providers should be aware that patients who are advised to stop their hormones before surgery may experience emotional and physical effects [61]. Common symptoms include mood swings and hot flashes. If patients stop their hormones for longer periods, this can result in reversal of the feminizing effects in MtFs. Despite the assumption that there is an increased perioperative cardiovascular risk in FtMs taking testosterone, to date, there is no conclusive evidence to support this. If FtMs stop their hormones for longer periods, this can result in reversal of the masculinizing effects, including loss of muscle mass and resumption of menses. Due to the fact that complete withdrawal of hormone treatments can have a profound impact on the patient, the surgeon and endocrinologist should collaborate in making decision on the use of hormones during the month before surgery and hormone therapy resumption after surgery. There are no documented drug-drug interactions among estrogen, the various androgen blockers and testosterone with anesthesia medications. Administration of anesthetic to transgender patients during the intraoperative period should proceed according to standard practice. Drug therapy after gonadectomy must respond to several conditions [62]. In FtMs, after hysterectomy and ovariectomy, the dose of testosterone should be decreased, up to halving the dosage, but never discontinuing it due to the risk of osteoporosis. In MtFs after orchiectomy, androgenic blockade should no longer be necessary. In MtF older patients (or patients with increased cardiovascular risk) it is suggested to reduce the estrogen dose to half the pre-operative dose. It is recommended that patients continue on estrogen indefinitely in order to preserve skeletal health; the reduced doses are such as to still maintain feminization. It may be that MtFs need a small amount of androgens to correct low libido, as is suggested for women with hypoactive sexual desire disorder. In general, most transsexual individuals indicate an improvement in their sex life and more sexual excitement after sex reassignment surgery
Actually, current data suggest a biologic origin for the etiology of transgender identity. Neuroanatomical studies provide the strongest evidence for the organic basis in this matter, but the sample sizes of most studies on this subject are small and the
conclusions must be interpreted with caution. Transgender care is a challenging, multidisciplinary, and developing field in medicine. The transgender population is rapidly growing and the existence of non-binary or gender queer genders gets increasingly more attention. Before the start of any type of therapy, the physician needs to discuss the pros and cons of the several treatment options so that the transgender individual can make a well-considered decision. GAHT is an indispensable medical intervention for many transsexuals, transgender and gender nonconforming people with GID. This therapy must be identified and calibrated on the basis of the patient’s objectives, the risk/benefit ratio of the drugs, the presence of other medical conditions, evaluating the various socio-economic problems. Gender dysphoria in adolescence is a condition associated with profound psychological suffering and in some cases with lethal ending. For this reason, the possibility of an early management, even of a pharmacological type, represents an important therapeutic option. Teenagers with gender inconsistency can benefit from that treatment to improve the quality of life, preventing the onset or exacerbation of associated psycho-pathologies. Because gender-related abuse is strongly associated with psychiatric distress degree during adolescence, denying the suppression of puberty and subsequent feminizing or virilizing hormone therapy is not a “neutral” option, but on the contrary it can be harmful, with serious consequences. Before initiation of GAHT fertility preservation is recommend. GAHT seems to be reasonably well tolerated when hormone levels are maintained within physiological ranges and presents a risk profile similar to that of sex hormone replacement therapy in biological men and women. Malignancies related to cross-sex hormone treatment of transsexuals have so far, fortunately, been a rare occurrence. The current available evidence does not show that GAHT increases oncologic risk for transgender individuals even if GAHT is relatively recent in medicine. Most transsexuals undergo treatment well before middle age and as part of that population start to age there is the implication of exposure to hormones over more than three or four decades. Transgender individuals should undergo cancer screening for all organs present regardless of transition status. In reality, trans people presents an increased overall mortality, but current data do not attribute this to GAHT but to nonhormone-related causes such as suicide, AIDS and drug abuse.
Funding: This research received no external funding
Informed Consent Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.Author contribution statements: M. Vetri, A. Cataldi and A. Naselli designed the study. A. Cataldi and A. Naselli analysed the data. M. Vetri and A. Vetri wrote the paper with input from all authors.
Approval of the manuscript: All authors read and approved the final version of the manuscript.
