Author(s): Radwa M hamed
Glioblastoma multiforme (GBM) remains the most aggressive brain tumor in adults despite the advances in diagnosis and treatment over the past few decades [1]. Standard treatment for patients with newly diagnosed GBM consists of maximal safe resection followed by postoperative radiation with concomitant and adjuvant temozolomide [2]. Despite that, recurrence is common and the prognosis remains poor with a median survival of 12?15 months [3].
Glioblastoma multiforme (GBM) is the most common and lethal adult brain tumor and contributes to high social and medical burden as a result of its aggressive nature and significant morbidity [4]. The optimal number of adjuvant temozolamide cycles remains controversial and depends on center experience and practice. Based on recent review, twelve cycles of adjuvant temozolomide slightly improved PFS with no improvement in OS compared to six cycles [5].
Treatment of recurrent GBM is a challenge as the treatment options are limited with modest activity [6]. At the time of recurrence, only a small number of patients with well-localized tumors are eligible for re-resection and literatures found that surgery increase morbidity in a recurrent setting [7]
Re-irradiation improves local disease control in a proportion of patients. However, it is not always feasible due to the hazards of cumulative neurotoxicity [8].
In the recent years different cytotoxic chemotherapy and targeted therapy have been used in patients with recurrent GBM improve disease control [9, 10]. Bevacizumab as a single agent or in combination with chemotherapy is the standard treatment offered in this clinical scenario [11]. However, in low- and middle-income countries, bevacizumab is not easy available due to financial constraints. Multiple drugs such as lomustine, temozolomide (TMZ), or PCV (procarbazine, lomustine, and vincristine) regimen are commonly used [6].
Temozolomide rechallenge has been studied in multiple clinical trials in various schedules and doses with mixed results [12, 13]. This drug has provided better overall survival (OS) in comparison to salvage PCV regimen [14].
A 50-year-old male presented with a two-month history of headache and convulsions. He was a non-alcoholic, non-smoker with no co-morbid medical or surgical importance. The condition started in March 2016. Magnetic resonance imaging (MRI) brain with contrast showed left parietal cortical SOL with imaging features which was highly suggestive of high grade glioma (2 x 2 x 2.9 cm).
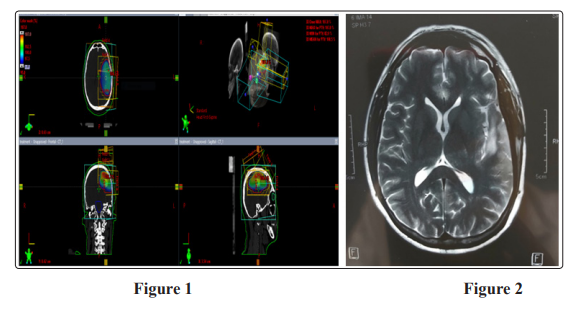
The patient underwent surgical excisional biopsy in April 2016. Pathology showed GBM WHO grade IV, received CCRTH 60 GY/ 30 Fx in 6 weeks (figure 1), with temozolomide 100 mg daily, started in May 2016 ended in July 2016 with good tolerance to radiation and chemotherapy, then received 6 cycles adjuvant temozolomide ended in Jan 2017 and kept under follow up with brain MRI every 3 months. At the first assessment postadjuvant tomozolomide, MRI show progression of the residual mass with newly developed neurological symptoms in the form weakness in right upper limb, magnetic resonance spectroscope (MRS) confirmed the progression with active residual tumor. Due to non availability of bevacizumab or access to MGMT test, temozolomide was re-challenged with the standard dose 150/ 200 mg/ m2 five days every four weeks with MRI assessment every 3 cycles and laboratory investigation every cycle. Due to good tolerance and response to treatment patient received it till October 2018, MRI brain with contrast October 2018 showed tiny enhancing foci 5 mm (figure 2). The patient continued another 3 months of temozolomide till Jan 2019 then stopped tomozolamide, as MRS showed no residual or recurrent lesion.
At April 2019, MRI brain with contrast showed Almost stationary appearance of the operative bed cortical altered signals yet with newly developed mass lesion noted at the left deep parietal periventricular regions involving the left globus pallidus measures about (2.1 x 2.6 x 2.2 cm) and perilesional edema (figure 3), MRS revealed dropped NAA with elevated Choline and creatinine, picture is likely of high grade glioma.
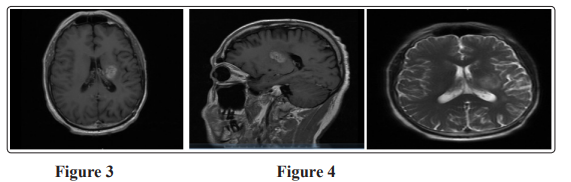
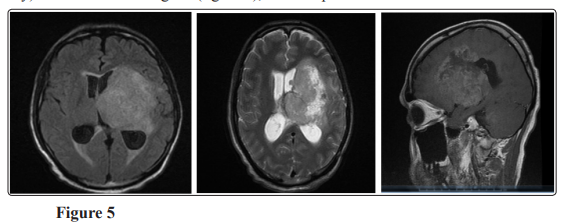
The patient started another three cycles of temozolomide; MRI brain with contrast in July 2019 showed mild increase in size of previously noted intra axial SOL 3 x 2.5 x 2.9 implicating the posterior limb of internal capsule (figure 4). The patient shifted to irrinotecan and bevacizumab but unfortunately after 2 cycles, MRI showed Progressive course of the size and extensions of the left deep parietal periventricular mass measuring about 6.5 x 5.5 x 7.2 cm Invading the midbrain with the left cerebral peduncle, pons (with patent basilar artery) and medulla oblongata. (figure 5), then the patient admitted to ICU due to DCL and died in October 2019.
Temozolomide rechallenge can be considered for patients who had a prolonged response to initial temozolomide treatment, with a long treatment free interval (TFI) after completion of adjuvant temozolomide. The cytotoxic effect of temozolomide strongly depends on O6-methylguanine DNA-methyl transferase (MGMT), which repairs DNA damage caused by the drug. Lowdose continuce temozolomide administration can decrease MGMT activity [13].
Currently there is no recommended duration or dosing schedule in recurrent cases. At first recurrence, temozolomide is active at a standard dose of 150 - 200 mg/m2, administered for five days every 4 weeks with PFS ranges (18%-24%) [15, 16].
Franceschi et al, documented that temozolomide rechallenge could prolong PFS and OS in patients with a TFI ≥5 months compared with nitrosurea (PFS 8.1 vs 5.8 months; p = 0.020, HR: 0.550; OS: 17.7 vs 11.6 months; p= 0.014; HR: 0.538; respectively), and this result is independent on MGMT methylation status [17].
In the RESCUE Phase II trial, patients who received continuous low dose temozolomide after at least an 8-week TFI achieved a longer PFS than those whose disease progressed during adjuvant TMZ (3.7 vs 1.8 months, respectively) [18].
Phase II DIRECTOR trial, compared two different dose-intense temozolomide schedules, patients with methylated MGMT had longer time to treatment failure and median survival if TFI was longer than 2 months compared with TFI shorter than 2 months(5.5 vs 1.8 months and 16.1 vs 11.3 months) [19].
Our patient had PFS 36 months after TFI of only 3 months and rechallenged with temozolomide in the standard dose 150-200 mg/m2 for 5 days every 4 months without MGMT test as it is not available in our center.
Recurrent GBM is a challenge to manage; the selection of the treatment modality requires a delicate balance between aggressiveness of treatment, outcome, cost of care and quality of life. Temozolomide rechallenge is highly dependent on response to adjuvant temozolomide but there is no standard treatment free interval cut off point to assess response temozolomide in first recurrence.
