Author(s): Richmond R Gomes
Melkersson-Rosenthal syndrome (MRS) is a rare neuro-mucocutaneous syndrome of unknown etiology with chronic and progressive course, and clinical diagnosis is usually by exclusion. Rarely, it presents with the full triad of recurrent or persistent facial swelling, relapsing facial palsy, and a fissured, geographic tongue. It is rarely possible to observe all aspects of the classical triad at the same time, since these symptoms may appear in different times of life cycle. Although etiology of MRS is unclear, various factors such as infections, genetic predisposition, immune deficiency, food intolerance and stress have been held responsible. The differential diagnosis of MRS includes also chronic inflammatory and infective diseases characterized by granulomatous infiltration, as well as rosacea, contact dermatitis, allergic reactions and Bell’s palsy. The therapeutic options are varied, though most commonly include corticosteroid therapy and nerve decompression surgery.
Melkersson Syndrome was first described in 1928 as peripheral facial paralysis and swelling of lips. In 1931, Rosenthal completed the triad by adding the presence of fissural tongue. The classical form of the Melkersson Rosenthal Syndrome (MRS) consists of the clinical triad of recurring facial nerve paralysis, swelling of one or both lips and fissural tongue [1]. Cases displaying the entire triad are very rare. However, oligosymptomatic or monosymptomatic forms of this syndrome outnumber those with the classic triad, which is found in around one-fourth or less of patients [2-4].
Melkersson-Rosenthal syndrome is an unusual neuromucocutaneous, granulomatous inflammation of the facial region of unknown etiology, which is probably caused by a neurovascular dysfunction that leads to hyperpermeability of the vessels and consequent facial edema. Age at onset varies from early childhood to late adulthood but its highest prevalence is among young female adults. There is also a dominant inheritance trait that explains the aetiopathogenesis of this characteristic [5,6].
Recurrent lip swelling, also termed Miescher’s syndrome or Miescher’s cheilitis granulomatosa (MCG), is the most common monosymptomatic presentation of MRS.Infectious conditions, including orofacial herpes, may precede the onset of MRS. Painless swelling, which is usually intermittent and fluctuant at the beginning, may become constant and the differential diagnosis of this sub-type of oro-facial granulomatosis includes angioedema(AE), contact dermatitis, Crohn’s disease, sarcoidosis, foreign body reaction and chronic, granulomatous infections. The characteristic histopathological features of MRS include granulomas with epithelioid cells, Langerhans type giant cells with multiple nuclei, perivascular mononuclear infiltration, non-caseating granulomas, lymphedema and fibrosis [7]. It can be difficult to detect these features; however, their absence should not exclude the MRS diagnosis [8,9]. Since MRS is a clinical syndrome, histopathologic findings are not required for diagnosis. While biopsy helps the diagnosis, it is also helpful in the differential diagnosis of Crohn’s disease and sarcoidosis [10]. Diagnosis is mostly based on clinical findings [1,11]. We present the case of a 32-year-old man with MRS who had partially responded to systemic corticosteroids.
A 32-year-old, male, nonsmoker Bangladeshi businessman, not known to have diabetes mellitus, hypertension, ischemic heart disease or bronchial asthma sought outpatient care with the complaints of sudden onset of left hemifacial paraesthesia and numbness for 3 days associated with inability to close his left eye for the same duration. He denied any headache, vomiting, fever, altered consciousness, convulsion, visual, speech, hearing or swallowing difficulties. He also had no history of joint pain, pain, shortness of breath or abnormal bowel habit. The current presentation had been preceded by a few blisters similar to those usually observed in herpes labialis but there was no mouth ulcer or lesion over external auditory meatus. He cited a similar episode of peripheral facial paralysis on the same side at 2 years back for which he received physiotherapy and conservative treatment, and the symptoms disappeared spontaneously. Background therapy included off and on taking of proton pump inhibitor with no personal and family history of adverse drug reactions, atopy, contact dermatitis, urticaria, angioedema, cranial nerve palsy, granulomatous, inflammatory or connective tissue diseases. On physical examination, he presented grade IV (House-Brackmann) lower motor neuron type facial palsy (figure 1), on the left side and marked left sided orofacial swelling (figure 2) and plicate tongue (figure 3). No lacrimal, conjunctival or palate alterations were observed. Other systematic examination including detailed neurological examination failed to reveal any abnormalities. On investigation, complete blood count, random blood sugar, chest x-ray, ANA, c-ANCA, p-ANCA were negative. Patient refused for histopathology and biopsy.
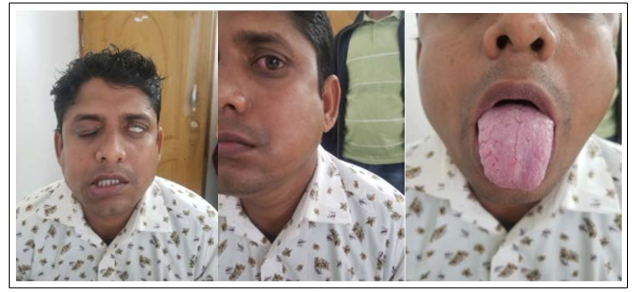
Figure 1, 2 & 3: Showing left sided lower motor neuron type facial palsy, left sided orofacial swelling and fissural tongue respectively
Faced with this triad of clinical manifestations, and in the absence of evidence suggesting other pathologies, the diagnosis of Melkersson-Rosenthal syndrome was established. The treatment instituted was corticosteroid therapy and neuromuscular physiotherapy, which provided gradual improvement. One month after of the onset of the condition, there is moderate improvement in the facial paralysis (House-Brackmann grade II) and reduction of faced with this triad of clinical manifestations, and in the absence of evidence suggesting other pathologies, the diagnosis of Melkersson-Rosenthal syndrome was established. The treatment instituted was corticosteroid therapy and neuromuscular physiotherapy, which provided gradual improvement. One month after of the onset of the condition, the patient presented moderate improvement in the facial paralysis (House-Brackmann grade II) and slight reduction of the orofacial swelling.
The most frequent etiology of facial paralysis is its idiopathic form (Bell’s palsy), which occurs in 80% of cases and generally presents complete resolution in most cases and without the occurrence of relapses. MRS is a rare disease of unknown etiology, chronic and progressive course, with neuro-mucocutaneous involvement characterized with a clinical triad consisting of permanent or recurring orofacial edema, recurring facial paralysis and fissural tongue. It has estimated incidence of 0.08% in the general population [12]. Onset of this disease is more frequent in young adults, between the second and the third decades of life [13]. Classical triad of MRS can only be observed in 8% to 18% of the patients, which makes it difficult to diagnose this condition. All clinical findings can be seen all at once or at different times. Presence of at least one of the findings of idiopathic facial paralysis or fissural tongue together with permanent or recurring orofacial edema is sufficient for MRS diagnosis [14-18].
The most frequent finding of MRS is acute, diffuse, painless and non-pitting orofacial edema syndrome, occurs in 80 to 100% of cases which is most frequently seen on lips [14,15,19,20]. The upper lip is involved more frequently although other areas of the face, such as the chin, cheeks, periorbital region, oral mucosa, tongue and gums, may be affected. Edema has a tendency for recurrence and usually disappears within few hours to few weeks [7]. MRS resembles angioedema, but it can be differentiated with the lack of response to antihistaminic drugs, tendency to last longer and by causing fibrosis in the involved tissues [21]. Fissures can be observed in the central area (central cheilitis), corners of the mouth (angular cheilitis) or other areas of the involved lip [22]. The second characteristic finding of MRS is facial paralysis which can be observed in 30% to 35% of the patients [23]. Paralysis can be transient, or can become permanent in time. It can be unilateral as well as bilateral or partial. The paralysis has been related either to the pressure of the edema on the nerve passing through the facial canal within the temporal bone or the granulomatous infiltration of the nerve [24,25]. The facial paralysis related to MRS may appear after months or years before or after the orofacial edema [21]. The third finding of MRS is the fissural tongue. Since fissural tongue is a widespread abnormality in the society, it is of lesser importance in the MRS diagnosis [26]. Other findings of MRS can be listed as trigeminal neuralgia, ocular palsy, tinnitus, paresthesia, dysfunction of the lacrimal and saliva glands, migraine, keratitis, increases of secretions and psychological episodes [24,27]. Although the disease can be controlled with treatment. it can recur in the future [28].
The predominant theory is that it is of multifactorial genesis, and MRS does not have specific biomarkers. Its clinical diagnosis is usually via exclusion. The observation of familial inheritance and the suggestion of a relationship with autosomal translocation t(9;21) (p11; p11) supports the hypothesis of genetic predisposition [29,30]. The proposed potential etiologies are based on the immunogenicity (allergy and autoimmunity - not yet proved) of the syndrome, associated infections, and the genetics of the patient. Morales et al found a lack of sensitization to food, additives, or contact in such cases [31]. In contrast, Fitzpatrick et al found that more than 50% of the patients had a positive allergy test, suggesting involvement of a type 1 hypersensitivity reaction in the development of orofacial granulomatosis [32]. The reported association between MRS and autoimmune thyroiditis as well as systemic lupus erythematosus favors the proposed theory of autoimmunity [33,34]. Crohn’s disease is the only autoimmune disorder that has shown a strong association with MRS. As orofacial granulomatosis has been proposed to be a subtype of Crohn’s disease, the authors recommended long-term follow-up regardless of the current absence of systemic symptoms [25,35].
Complement deficiency was reported in a few MRS cases. Freeman reported an associated deficiency of C1q along with a low-normal level of C4 and a positive pricked skin test [36]. Troiano et al explored the role of infections and genetics in the MRS etiology. The two main pathogens investigated were Mycobacterium tuberculosis and Borrelia burgdorferi. The skin biopsies were positive for M. tuberculosis but controversial for B. burgdorferi [37]. Trials of treating MRS with anti-tuberculosis regimens have not appeared in literature. Malignancy was reported in six cases of MRS, half of which were hematologic. They comprised malignant lymphogranulomatosis, Hodgkin’s disease, leukemic macrocheilitis, squamous cell carcinoma, retroperitoneal liposarcoma, and malignant pharyngeal lymphoma [36,38-42].
MRS can be frequently diagnosed by clinical criteria with no need for further investigation [43,44]. It is well known, on the other hand, that there is no definitive therapy for MRS and recurrences are frequent [2,45]. The good response of MRS to steroids and immunosuppressive agents is consistent with the proposed role of autoimmunity and allergy in the syndrome. Liu and Yu recommended systemic corticosteroids as the first line of treatment [46]. Rivera-Serrano et al agreed with others recommending the use of intralesional injections to avoid the risk of side effects [47,44,48]. Sobjanek et al suggested that combination therapy of intralesional corticosteroids and dapsone (the former enhancing the efficacy of dapsone) was safe and effective treatment [49]. Although intralesional corticosteroid may alleviate cheilitis granulomatosa, its impact on facial palsy remains uncertain.
If all else fails, Stein et al recommended anti-tumor necrosis factor α agents, which they used successfully to treat a patient with noncaseating granulomatous cheilitis with neurological impairment and elevated Ig who had responded to adalimumab after failing steroids, azathioprine, and methotrexate [50]. Antibiotics with an anti-inflammatory function, (e.g., minocycline) were also used in cases of granulomatous cheilitis [51,52]. Ratzinger et al, who documented a good outcome with clofazimine and infliximab, recommended these agents for treating MRS with or without Crohn’s disease [53]. Because of the serious side effects of biological agents, however, we recommend that they be reserved for patients in whom other agents have failed or who present with systemic involvement. Long-term follow-up visits are also recommended at this stage to observe the patient’s response to treatment as well as to identify any relapses that might occur. Finally, a surgical approach has been suggested only for those patients with oro-facial swelling refractory to steroid therapy and/ or who present a significant face deformation [2]. and infliximab. However, the clinical course and therapeutic response of patients can be unpredictable, and, on many occasions, there may be spontaneous remissions or recurrences [54].
Melkersson-Rosenthal syndrome (MRS) is a relevant disease, poorly recognized and sometimes undiagnosed that can cause social and functional damage to affected individuals. It may present as a classic triad of orofacial swelling, facial palsy and fissured tongue or, more frequently, with oligo/mono-symptomatic features. Differential diagnosis with other granulomatous diseases and angioedema must be considered, as symptoms and signs usually overlap. No specific biomarkers for MRS exist and clinical diagnosis is often of exclusion. An individualized and multidisciplinary approach is fundamental in the treatment of the disease in order to avoid complications and permanent sequelae.
