Author(s): Akintoyese O Alabi*, Beatrice O T Ifesan, Opeyemi l Alabi, Ngozi I Akosu
This study described the α-Glucosidase, α-amylase, antioxidant activity, and glycemic index of wheat - Phyllantus amarus cookies, using standard methods. It was also observed that these medicinal plants have the ability to inhibit α–amylase, α-glucosidase and reducing effect on the radical DPPH and glycemic index of the formulated cookies. The microbial count on Phyllantus amarus cookies showed that only total viable count between (2-5) 103 cfu/g shows re-sponse against the four samples tested, while Staphylococcus aureus (cfu/g), Coliform count (cfu/g), Fungi count (sfu/g) showed no response which is an ev-idence that these samples may be good for consumption. Physical characteris-tics; thickness, diameter, weight, spread ratio and hardness were also observed which ranged from 12.68mm- 14.37mm, 2.76mm - 2.90mm, 4.18g - 6.44g, 0.17- 0.21 and 8.13mm - 11.57mm respectively. The moisture, protein, ash, fat, fibre, carbohydrate contents of the control cookies (100% Wheat flour) were 5.08%, 14.60%, 1.63%, 20.75%, 1.78%, 57.95% respectively while that of wheat-Phyllantus amarus cookies ranged from 4.14 %- 4.83 %, 13.95 % - 15.92 %, 1.22 % - 2.15 %, 19.83 % - 21.40 %, 2.70 % - 3.38 %, 56.64 % - 60.38 % respectively.
Diabetes mellitus (DM), a chronic metabolic disorder characterized by high blood glucose levels, continues to be a major medical concern worldwide due to its high prevalence and potential deleterious effects. It includes a group of met-abolic diseases characterized by hyperglycemia, in which blood sugar levels are elevated either because the pancreas do not produce enough insulin or cells do not respond to the produced insulin [1]. Therefore a therapeutic approach to treat diabetes is to decrease postprandial hyperglycemia [2]. One goal of ther-apy for diabetic patients, especially type 2, is the maintenance of normal blood glucose levels after meal [3]. Postprandial hyperglycemia plays an important role in the development of type 2 diabetes and its complications. One of the therapeutic approaches for decreasing of blood glucose rise after a meal is to retard the absorption glucose by inhibition of carbohydrate hydrolyzing en-zymes such as α-amylase and α-glucosidase and also a low glycemic index (GI) diet improves certain metabolic consequences of insulin resistance, glucose and lipid metabolism [3-5].
Diabetes mellitus is a chronic endocrine disorder that affects the metabolism of carbohydrates, proteins, fat, electrolytes and water. It includes a group of met-abolic diseases characterized by hyperglycemia, in which blood sugar levels are elevated either because the pancreas do not produce enough insulin or cells do not respond to the produced insulin [1]. For a long time natural products from plants have been used for the treatment of diabetes, mainly in developing coun-tries where the resources are limited and affordability and access to modern treatment is a problem [6].
Phyllanthus amarus (Schum and Thonn) is one of the most pharmacologically important species of the Phyllanthus family. It is a medicinally important plant belonging to Euphorbiaceae otherwise known as ?stone breaker?, ?carry me seed? etc. In Nigeria, the plant is called ?geron tsuntsaye? (Hausa), ?eyin-olobe? (Yoruba) and ?ngwu? (Igbo). Phyllanthus amarus is an erect annual herb of not more than one and half feet tall. It has small leaves and yel-low flowers. It is commonly found in forest areas, arid land, savannah areas, leached and exhausted soil in many countries including China [7].
The decoctions of various parts of the herbs are used traditionally for the treatment of hepatic, urinary and sexually transmitted diseases, diabetes, hy-pertension and cancer. In folk medicine, P. amarus herb has found applica-tions in the management of several health problems such as diarrhea, dysentery, dropsy, jaundice, intermittent fevers, urinogenital disorders, sca-bies and wounds. Other reported pharmacological activities of the plant include anticancer, antioxidant, antileptospiral, antimicrobial, anti-diabetic, anti-inflammatory and anti-convulsant activities [8].
Freshly harvested leaves of Phyllatus amarus and were obtained from the teaching research farm of The Federal University of Technology Akure Ondo State. It was identified at the Department of Crop, Soil and Pest, Federal Uni-versity of Technology Akure Ondo State.
All chemicals and reagents used in this study were of analytical grade and water was glass distilled.
Four samples of cookies were formulated as presented in Table 3.1, where varying percentage of wheat flour was substituted with the composite leave flour comprising of phyllantus amarus (PA) leaf. The formulations include; the control cookies with 100 g of wheat flour with 0g of leave flour (PA), cookies with 95 g of wheat flour to 5 g of leave flour (PA), cookies with 90 g of wheat flour to 10 g of leave flour (PA), and cookies with 85 g of wheat flour to 15 g of leave flour (PA). Cookies was produced as described by as shown in figure 3.1, using flour (wheat and leave flour), sugar, margarine, salt, sodium bicar-bonate, water, milk and vanilla essence which were all weighed appropriately and the two stage creaming up method was used [10]. All the ingredients ex-cept flour were mixed thoroughly in a Kenwood mixer (a 3-speed hand mixer), it was then transferred into a bowl and the flour and sodium bicarbonate was added with continuous mixing for 15 minutes until smooth dough was ob-tained. A piece of this dough was cut, placed on a clean platform and then rolled out using rolling pin until the desired uniform texture and thickness was obtained. Cookies cutter was used to cut the sheet of the dough into the re-quired shapes and sizes. These were then transferred on to a greased (with margarine) baking tray. The baking was done at 200 °C for 15-20 minutes, af-ter which the hot cookies were removed from the pan and placed on a clean tray for cooling. The cookies were then packed after scooling in polyethylene sachets of appropriate thickness and permeability, sealed and then kept at room temperature for further analysis.
| Ingredients | CWPA 1 | CWPA2 | CWPA3 | CWPA4 |
|---|---|---|---|---|
| Wheat flour (g) | 100 | 95 | 90 | 85 |
| Leave flour (g) | 0 | 5 | 10 | 15 |
| Sugar (g) | 10 | 10 | 10 | 10 |
| Margarine (g) | 30 | 30 | 30 | 30 |
| Salt (g) | 2 | 2 | 2 | 2 |
| Sodium bi-carbonate (g) | 1 | 1 | 1 | 1 |
| Milk (g) | 10 | 10 | 10 | 10 |
| Water (ml) | 50 | 50 | 50 | 50 |
| Vanilla es-sence (ml) | 2 | 2 | 2 | 2 |
CWPA 1
= cookies with 100 g of wheat flour and no plant leave
flour (control sample)
CWPA 2
= cookies with 95 g of wheat flour and 5 g of plant leave
flour (sample 1)
CWPA 3
= cookies with 90 g of wheat flour and 10 g of plant leave
flour (sample 2)
CWPA 4
= cookies with 85 g of wheat flour and 15 g of plant leave
flour (sample 3) Source [10].
Figure 3.1: Flow Diagram of Cookies Production [10].
Porcine pancreatic α-amylase (PPA; A05329G191; 1ml) was dissolved in 9ml of 20mM phosphate buffer (pH 6.9) to give 4 Unit/mL solutions. The stock solution of the plant extracts was prepared by dissolving 1g of the extract in 5mL of 2% DMSO to give a concentration of 20mg/ml. Potato starch (0.5% w/v) was dissolved in 20mM phosphate buffered saline (pH 6.9) and placed in a boiling water bath to get a clear solution. The alphaamylase inhibition assay was done using the chromogenic nonpre-incubation method adapted from Sigma-Aldrich. Briefly, 40μL of plant extract, 160μl of distilled water, and 400μL of starch solution were mixed in a screw top plastic tube. The reaction started by the addition of 200μl of the enzyme solution and the tubes were in-cubated at 25°C for 3min at room temperature. The enzyme solution was add-ed at 1min interval from the start of the reaction. Briefly, 200μl of the mixture was withdrawn into a separate test tube containing 100μl of DNS color rea-gent (50.68g sodium potassium tartrate dissolved in 70ml of 2M NaOH with 0.026mM of 3,5-dinitrosalicylic acid) and placed in a water bath maintained at 85-90°C for 15min. The mixture in each tube was diluted with 900mL of dis-tilled water and the absorbance was measured at 540nm. For each concentra-tion of the extract used, blank incubation was prepared by replacing the en-zyme solution with distilled water (200μl) at the start of the reaction, to cor-rect for the absorbance generated by the plant extract. Control incubations, representing 100% enzyme activity, were carried out in a similar manner by replacing plant extract with 40μL of 2% DMSO. All the tests were run in trip-licate. From the value obtained, the percentage (w/v) of maltose generated was calculated from the equation obtained from the maltose standard calibration curve (0-0.1% w/v maltose). The level of inhibition was calculated as follows: Inhibition (%) = 100-% reaction (at min), where % reaction = mean maltose in sample x 100/mean maltose in control [11].
The effect of the plant extracts on α-glucosidase activity was determined ac-cording to the method described by Using α-glucosidase from Saccharomyces cerevisiae [12]. The substrate solution p-nitro phenyl glucopyranoside (pNPG) was prepared in 20mM phosphate buffer, pH 6.9. 100μL of α-glucosidase (0.3U/ mL) (1-2mg α-glucosidase was dissolved in 100ml of phosphate buffer pH 6.8 containing 200mg BSA) was pre-incubated with 50μL of the sample for 10min. Then 50μL of 3.0mM (pNPG) as a substrate dissolved in 20mM phosphate buffer (pH 6.9) was then added to start the reaction. The reaction mixture was incubated at 37°C for 20min and stopped by adding 2mL of 0.1M Na2 CO3 . The -glucosidase activity was determined by measuring the yellow-colored paranitrophenol released from pNPG at 405nm.
In vitro starch hydrolysis rate and GI were determined according to Cookies samples (50 mg) were incubated with 1 mg of pepsin in 10 ml HCl-KCl buffer (pH 1.5) at 400 °C for 60 minutes in a shaking water bath [13]. The digest was diluted to 25 ml by adding phosphate buffer (pH 6.9), and then 5 ml of a-amylase solution containing 0.005 g of a-amylase in 10 ml of buffer was add-ed. The samples were incubated at 37 °C in a shaking water bath. 0.1 ml of the sample was taken from each flask and boiled for 15 minutes to inactivate the enzyme. Sodium acetate buffer (1 ml 0.4 M, pH 4.75) was added and the resid-ual starch digested to glucose by adding 30 ml amyloglucosidase and incubated at 60 °C for 45 mins. Glucose concentration was determined by adding 200 ml of dinitrosalicylic acid colour reagent. The reaction mixtures was stopped by placing the tubes in a water bath at 100 °C for 5 minutes and then cooled to room temperature. The reaction mixture was then diluted by adding 5 ml of distilled water and the mixture was centrifuged at 1200 rmp. The supernatant was collected and the absorbance measured at 540 nm using spectrophotome-ter. The rate of starch digestion was expressed as the percentage of starch hy-drolyzed. A 50 mg sample of glucose was used as the standard.
The weight (W) of the cookies was measured on a weighing balance. The diam-eter (D), thickness (T) and spread ratio were all analyzed as described by [14]. For the determination of cookies diameter (D), four cookies were placed edge to edge. The total diameter of the six randomly selected cookies was measured in mm by using a ruler. The cookies were rotated at an angle of 90° for dupli-cate reading. The average diameter was reported in mm. To determine the thickness (T), four cookies were placed on top of another and the total height was measured in mm with the help of a ruler. This was repeated thrice to get an average value and results were reported in mm. Spread ratio was deter-mined with the help of following formula:
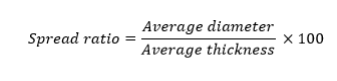
Sensory evaluation was performed using the modified method of [15]. 40 students were used from the Department of Food Science and Technology of The Federal University of Technology, Aku-re. The samples were checked for Appearance, Aroma, Taste, Texture, Mouldability and Overall Acceptability. The participants evaluated the samples using a 9 point hedonic scale quality analy-sis with 9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like slightly, 5 = Neither like or disliked, 4 = Dislike slightly, 3 = dislike moderately, 2 = Dislike very much, 1 = Dislike extremely.
The media used in this research work were: Nutrient Agar (NA), MacConkey Agar (MCA), Mannitol Salt Agar (MSA) and Potato Dextrose Agar (PDA). They were prepared according to the manufacturer’s specification as directed on the containers.
Glass ware such as conical flask, measuring cylinders, Petri-dishes, McCartney bottles, cork borer and other glass container were washed, drained and dried. They were then autoclaved (121°C for 15 min) for sterilization, and were al-lowed to cool to 40 °C before used. Nutrient Agar (NA), Maconkey Agar (MCA), Monitor Salt Agar (MSA) and Potato Dextrose Agar (PDA) were also sterilized by autoclaving. Work surfaces were sterilized by swabbing with 95% ethanol. Aseptic working environment was achieved with the use of spirit lamp.
For every 100 ml of distilled water, 0.85 g of NaCl was weighed into it and dissolved by shaking. And 9 ml of the diluent was dispensed into serial dilu-tion bottles and sterilized for serial dillutions.
Twenty five millilitres of each cookies samples was added to 225ml of sterile saline water. This was thoroughly mixed in the bottle and pour plate method was used. Each sample (1ml) was transferred into sterile petri dishes and indi-vidual sterilized agar was poured and swirled to cover the surface of the petri dish. The coliform count and total viable count plates were incubated at 320C for 24h and the microbial growths were counted using colony counter. The to-tal count plates were incubated at room temperature for 72h [16].
Data were to analysis of variance (ANOVA). Comparison of means was car-ried out by Duncan’s multiple range test [17]. Statistical analysis was per-formed using the statistical package for social sciences (SPSS 17.0) and the means ± SD were calculated from triplicate determinations.
The proximate composition of cookies are represented on Table 1. The mois-ture content is between the range of (5.08% - 4.83%) with the highest value in sample S1 (5.08%) and the lowest value in sample S2 (4.83%). The moisture content of cookies production usually dependent on the time and temperature used for the baking process and the moisture content got from this study was found to be within the acceptable range for baked goods with respect to stor-age stability and microbial contamination.
The results of the Ash content shows that the value was between (1.22 - 2.15%) with the highest value from S3 (2.15%) and the lowest value in S4 (1.22%). However there was linear relationship (P > 0.05) between the activi-ties of sample S1 (1.63%) and sample S2 (1.73 %).
It was observed that the crude fibre content in the formulated cookies increased with increase in the amount of Phyllantus amarus cookies (0g, 5g, and 10g). Samples 1 (1.78%), sample 2 (3.00%) and sample 3 (3.38%), but there was a decrease in sample S4 (2.70%). The low crude fibre content in these samples are advantageous in absorption of glucose and fat as studies shown that crude fibre aids in reducing peaks of blood glucose following a meal due to delayed gastric emptying [18].
The crude protein in the formulated cookies was between (13.95% - 15.92%), with the highest value (15.92%) in sample (S4) and the lowest value (13.95%) in sample (S2). There was increase in the protein content of sample S2 (13.95 %), S3 (15.10%) and S4 (15.92%) as observed with increase in the amount of Phyllantus amarus flour (5g, 10g and 15g) added to the formulated cookies. Hence the Phyllantus amarus flour could be a moderate source of protein sug-gested that protein from plant sources have lower quantity, but their combina-tion with many other sources of protein such as animal protein may result in equivalent nutritional value [19]. The recommended dietary allowance (RDA) for protein is 56g for individual weighing 70kg and 46kg for adult weighing 50kg, and children 2g/kg/ day.
The carbohydrate content of formulated cookies was (56.64% - 60.38%). There was significant decrease in the carbohydrate content with increase in the amount of Phyllantus amarus flour added in sample S2 (60.38%), S3 (57.20%) and S4 (56.64%) respectively observed that some carbohydrate containing foods have a rapid rise followed by a slow decline in blood glucose concentra-tion [19]. This may also be one of the contributing factors for the efficacy of Phyllantus amarus plant as an antidiabetic agent [20].
The crude lipids content was between (19.83% - 21.40%). Lipids provide ex-cellent source of energy and enhance transport of fat soluble vitamins, insulate and protect internal tissues and contribute to vital cell processes. It has been suggested that enough lipid should be included in the diet to account for at least 20- 25% of the total caloric intake [18].
| Sample | Moisture content | Protein | Ash | Fa | Fibre | Carbohydrate |
|---|---|---|---|---|---|---|
| S1 | 5.08 ± 0.11a | 14.60 ± 0.21c | 1.63 ±0.35b | 20.75 ± 0.00ab | 1.78 ± 0.31b | 57.95 ± 0.35b |
| S2 | 4.14 ± 0.11b | 13.95 ± 0.07d | 1.73 ± 0.35b | 19.83 ± 0.11b | 3.00 ± 0.14a | 60.38 ± 0.35a |
| S3 | 4.84 ± 0.11a | 15.10 ± 0.21b | 2.15 ± 0.14a | 20.73 ± 0.39aab | 3.38 ± 0.18a | 57.20 ± 0.35b |
| S4 | 4.83 ± 0.35 a | 15.92 ± 0.11a | 1.22 ± 0.28c | 21.40 ± 0.71a | 2.70 ± 0.28a | 56.64 ± 0.81b |
Where;
S1 = Sample 1, 100% Wheat Flours, 0% Flour
S2 = Sample 2, 95% Wheat Flour, 5% leaves.
S3 = Sample 3, 90 % Wheat Flour, 10 % leaves.
S4 = Sample 4, 85% Wheat Flour, 15% leaves.
Several studies have reported the ability of various medicinal plants in inhibi-tion of α-amylase to be an important area of active research [21]. Also the eth-anol extracts of phyllantus amarus have also been reported to possess α- amyl-ase inhibitory activity in vitro [22].
The effect of wheat - P. amarus cookies on α-amylase enzyme presented in Fig-ure 1 shows that sample (4) 61.89 % had the highest inhibitory effect on α-amylase enzyme, with respect to the formulated cookies, there was a little de-crease in the inhibitory effect on α-amylase enzyme with decrease in the amount of Phyllantus amarus cookies 10g, 5g and 0g of sample (3) 59.87 %, sample (2) 58.67% and sample (1) 26.25% respectively. This is in line with the findings of who reported that nephroprotective and cardioprotective effect of Phyllanthus amarus extract in methanol caused a significant dose dependent decrease in the levels of total protein, cholesterol, alkaline and acid phospha-tases [23].
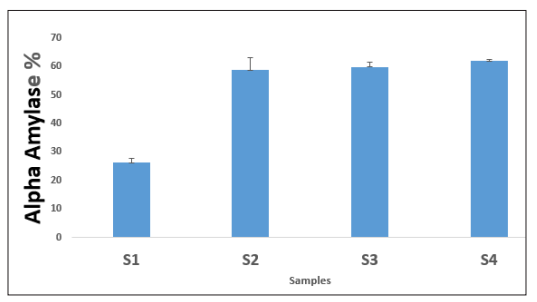
Figure 1: Alpha- Amylase Inhibition (%) of Wheat - Phyllantus Amarus CookiesWhere;
S1 = 100: 0, 100% Wheat Flours, 0% Flour
S2 = 95: 5, 95% Wheat Flour, 5% leaves.
S3 = 90: 10, 90% Wheat Flour, 10% leaves
S4 = 85: 15, 85% Wheat Flour, 15% leaves
Acarbose an α- glucosidase inhibitor is the first line drug for reducing post-prandial blood glucose in diabetic patients and was reported to reduce the rela-tive risk of cardiovascular event in patients with impaired glucose tolerance and type 2 diabetics [24]. However, researcher have found that P. amarus ash showed α- glucosidase inhibitory activity with nearly the same potency as ‘Acarbose’ when considered by the maximum percent inhibition and ICx 2 [25].
The P. amarus cookies showed the highest inhibitory effect of 82.58% on α-glucosidase enzyme in sample 4 (S4 ) followed by the cookies formulated with 10g of Phyllantus amarus flour (S3 ) 41.28%, the cookies formulated with 5g of Phyllantus amarus cokies (S 2 ) 66.10%, and the control cookies (S 1 ) 21.28% as represented in Figure 2. The high inhibitory effect of the Phyllantus amarus cookies obtained in this study is in agreement with the findings of who report-ed that Phyllantus amarus may play a role in decreasing glucose absorption which may be due to the inhibition of α- glucosidase activity in the intestine [19].
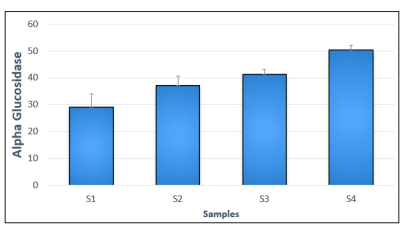
Figure 2: Alpha Glucosidase (%) of Wheat - Phyllantus Amarus Cookies
Where;
S1 = 100: 0, 100% Wheat Flours, 0% Flour
S2 = 95: 5, 95% Wheat Flour, 5% leaves.
S3 = 90: 10, 90% Wheat Flour, 10% leaves
S4 = 85: 15, 85% Wheat Flour, 15% leaves
A number of studies have shown that a low glycemic index (GI) diet improves certain metabolic consequences of insulin resistance, glucose and lipid metabo-lism [5]. The glycemic index is a scale that ranks the number of carbohydrates in foods from 0 to 100, indicating how quickly a food causes an individual’s sugar to rise. Carbohydrates are usually grouped according to their glucose postprandial responses, which are commonly classified in terms of high (>70%), medium (56-69%) and low (<55) glycemic index (GI). The postpran-dial blood glucose levels and the insulin response are dependent on the quality as well as the quantity of carbohydrates intake [26].
The in-vitro glycaemic index of P. amarus cookies is presented in Figure 3 which shows that the sample 4 (S 4 ) had the lowest glycaemic index of 50.76% while the control cookies sample (S1 ) had the highest glycaemic index of 72.01%. However, the glycaemic index of the cookies formulated with 5g, 10g and 15g of P. amarus cookies are (S2 ) 56.04%, (S3 ) 55.75% and (S 4 ) 50. 76% respectively, which showed there were a significant decrease in the glycaemic index of the formulated cookies with increase in the amount of P. amarus flour. This support the findings of Parikh, and who suggested that low glycemic in-dex diets may preserve high density lipoprotein (HDL) cholesterol and have a potentially positive effect in reducing coronary heart disease CHD risk [27]. Cookies formulated with 15g of P. amarus flour can be classified as a low gly-cemic index, having fall below 55 i.e (low (<55) glycemic index (GI) scale. T
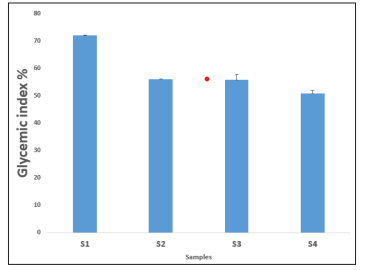
Figure 3: Glycemic Index (%) of Wheat - Phyllantus Amarus Cookies
Where;
S1 = 100: 0, 100% Wheat Flours, 0% Flour
S2 = 95: 5, 95% Wheat Flour, 5% leaves.
S3 = 90: 10, 90% Wheat Flour, 10% leaves
S4 = 85: 15, 85% Wheat Flour, 15% leaves
The mean values of the weight, diameter, height, spread ratio, ?E Total colour difference, L* lightness coordinate, a* red (+)/green (-) colour attribute, and b* yellow (+)/blue (-) colour attribute, of the formulated cookies are represented in Table 2. A significant increase in the thickness of the control cookies sample (S 1 ) 14.37g was observed compared to the cookies formulated with 5g, 10g and 15g P. amarus cookies sample; (S 2 ) 13.85g, (S 3 ) 13.19g and (S 4 ) 12.68g re-spectively.
The result obtained showed that there was no significant difference (P > 0.05) between the diameter of the control cookies (S 1 ) 2.90cm, with the cookies for-mulated with 5g, 10, and 15g phyllantus amarus cookies of sample (S2 ) 2.76cm, (S3 ) 2.87cm and (S4 ) 2.80cm respectively. The Weight (g) of the control cookies sample (S1 ) 6.44g showed a significantly decrease compared to the formulated cookies with 5g phyllantus amarus cookies (S2 ) 4.18g. However there was an increase in cookies formulated with 10, and 15, cookies of sample (S3 ) 5.16g, and (S4 ) 5.41g respectively. Spread ratio showed a progressive increase with corresponding increase in the amount of P. amarus added to the cookies and this is in-line with the documen-tation of Singh et al, who reported an increase in spread ratio of cookies with increase in non-wheat protein content [28]. However, there was no significant difference (P > 0.05) between the control cookies sample (S1 ) 0.17cm and the cookies with 5g of phyllantus amarus cookies (S2 ) 0.21cm and also between the cookies with 10g of P. amarus cookies (S3 ) 0.21cm and the cookies with 15g of Phyllantus amarus cookies (S4) 0.20cm.
A significant increase in the hardness (cm) of the control cookies sample (S1 ) 8.17g was observed compared to the cookies formulated with 5g, of sample (S2 ) 11.57g, however there was decrease in the thickness of the cookies with increase in the amount of pyhllantus amarus cookies incorporated with (S3 ) 8.13g and (S4 ) 10.00g respectively .Colour is an important quality attribute in the food and bioprocess industries, and it influences consumer’s choice and preferences. Food colour is governed by the chemical, biochemical, microbial and physical changes which occur during growth, maturation, postharvest handling and processing [29]. An increase in (a*) red(+)/green(-) colour at-tribute was observed in the control cookies sample (8.38) compared to the formulated cookies with 5g, 10g and 15g, phyllantus amarus cookies sample; (S2) 7.36, (S3) 5.69 and (S4) 5.06 respectively.
In the CIELAB colour system, parameter a* measuresd the red and green col-oration of the sample [29]. The lightness coordinate (L*) of the control cookies sample 65.17 showed a significantly increase compared to the formulated cookies with 5g, 10g, and 15g of phyllantus amarus cookies of sample (S2) 48.98, (S3) 43.93, (S4) 42.88 respectively. Also there is an increase in the con-trol cookies of sample (S1) 21.8 of b* yellow (+)/blue(-) colour attribute, compared to the formulated cookies with 5g, 10g, and 15g of phyllantus amarus cookies of sample (S2) 18.23, (S3) 19.9, (S4) 18.5 respectively. However, there was a progressive increase in the (b*) of the cookies with increase in the amount of phyllantus amarus cookies incorporated. The +?E total colour differ-ence in the control cookies showed no response, while there was an increase in the cookies formulated with 5g, 10g, and 15g of P. amarus (S2) 16.63, (S3) 21.50, (S4) 22.77 respectively
| SAMPLE | Thickness (mm) | Diameter (mm) | Weight(g) | Spread Ratio | Hardness (mm) | L* | a* | b* | ?E* |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 14.37±0.02a | 2.90±0.10a | 6.44±0.58a | 0.17±0.06a | 8.17±1.25b | 65.17±0.35a | 8.38 ± 0.17a | 21.83 ± 0.65a | - |
| S2 | 13.19±0.49c | 2.76±0.51a | 4.18±0.46c | 0.21±0.01a | 11.57± 0.81a | 48.98±0.21b | 7.36 ± 0.2b | 18.23± 0.01b | 16.63 ± 0.06d |
| S3 | 13.85±0.15b | 2.87±0.12a | 5.41±0.67b | 0.21±0.01a | 8.13±1.66b | 43.93±0.01c | 5.69 ± 0.27c | 19.92 ± 0.11a | 21.50 ± 0.05b |
| S4 | 12.68±0.25d | 2.80±0.10a | 5.16±0.28b | 0.20±0.36a | 10.00±0.36ab | 42.88±0.59d | 5.06 ± 0.15d | 18.56 ± 0.02d | 22.77 ±0.54 a |
WHERE = a * CIE red (+)/green (-) colour attribute. b* CIE yellow(+)/blue(-) colour attribute. L* CIE light-ness coordinate. ?E Total colour difference
Value are means ± standard deviation of three determinations. Values with different superscripts along the columns are significantly different (p<0.05)
Where; S1 = 100: 0, 100% Wheat Flours, 0% Flour. S2 = 95: 5, 95% Wheat Flour, 5% leaves. S3 = 90: 10, 90% Wheat Flour, 10% leaves S4 = 85: 15, 85% Wheat Flour, 15% leaves
The sensory attributes evaluated in the cookies made from wheat- Phyllantus amarus flour is shown in table 3. The results of the appearance shows that the value range between (6.17-8.10) with the highest value from Sample (1) the control and the lowest value in Sample (4). It was revealed from the result that the appearance of the sample (S1) was preferred by the panelist being the control cookies (S1) 8.10 compared to the cookies formulated with 5g, 10g and 15g of P. amarus flour ranging from (7.10 - 6.17). This could be attributed to the development of a minor bitter taste and a dark colour resulting from the addition of P. amarus flour which produced a difference from the regular dark brownies colour. Appearance has been a fundamental sensory attribute which has the ability to influence consumer acceptability [30].The aroma recorded the highest value in sample 1 (8.07) followed by sample 4 (6.57) which has the lowest value. Also the taste ranged between (5.87-7.83) with the highest value (7.87) in sample 1 and the lowest value (5.87) in sample 4. The gradual chang-es from both the taste and the aroma could be as a result of gradual incorpora-tion of the Phyllanthus amarus flour into the cookies ranging from 5g, 10g, and 15g.
The texture ranged between (6.60-7.50). There was a gradu.al changes from the cookies formulated with 5g, 10g, and 15g of P. amarus flour respectively. These may be attributed to increase in the fibre content of the formulated cook-ies due to the addition of Phyllanthus amarus flour which in turn transformed the degree of smoothness of the cookies and brought about a noticeable coarseness.
The result of the crispness was between (6.53 - 7.63) with the highest value (7.63) in sample S1 while the lowest value (6.53) was found in sample S4
.The overall acceptability of the control cookies sample (S1) 7.33 was signifi-cantly different (P < 0.05) from cookies formulated with 5g, 10g and 15g of P. amarus flour ranging from (6.90 - 7.00) which indicate that the consumer ac-ceptability of P. amarus cookies were generally above average.
| SAMPLE | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Appearance | 8.10±0.66a | 7.10±0.91abc | 6.73±1.20ab | 6.17±1.45bc |
| Aroma | 8.07±0.94a | 6.83±1.18bc | 7.07±1.20a | 6.57±1.48ab |
| Taste | 7.83±1.05ab | 6.60±1.04c | 6.23±1.14b | 5.87±1.48c |
| Texture | 7.50±1.10b | 7.00±0.95bc | 6.73±0.94ab | 6.60±1.14ab |
| Crispness | 7.63±1.03ab | 7.57±0.73a | 6.97±1.07a | 6.53±1.14ab |
| Overall Acceptability | 7.33±1.06b | 7.27±0.91ab | 7.00±0.74a | 6.90±0.91 c |
Where;
S1 = Sample 1, 100% Wheat Flours, 0% Flour
S2 = Sample 2, 95% Wheat Flour, 5% leaves.
S3 = Sample 3, 90 % Wheat Flour, 10 % leaves.
S4 = Sample 4, 85% Wheat Flour, 15% leaves.
Microbial count on cookies are presented in Table 4. The viable count (cfu/g) ranged between (5-2) 103 cfu/g, as sample 1, (2x103 ) has the highest value while sample 2, (2x10 3 ), sample 3, (2x103 ) and sample 4 have similar value. It was also observed that there were on growth on Staphylococcus aureus (cfu/g) ,coliform count (cfu/g), Funga (sfu/g) count. This may be an indication that the cookies are safe for consumption.
| SAMPLE | Total viable count (cfu/g) | Staphylococcus aureus (cfu/g) | Coliform count cfu/g | Funga count sfu/g |
|---|---|---|---|---|
| S1 | 5.00x103 | Nil | Nil | Nil |
| S2 | 2.00x103 | Nil | Nil | Nil |
| S3 | 2.00x103 | Nil | Nil | Nil |
| S4 | 2.00x10 3 | Nil | Nil | Nil |
Where;
S1 = 100: 0, 100% Wheat Flours, 0% Flour
S2 = 95: 5, 95% Wheat Flour, 5% leaves.
S3 = 90: 10, 90% Wheat Flour, 10% leaves
S4 = 85: 15, 85% Wheat Flour, 15% leaves
The obtained results highlight the high activities of Phyllantus amarus cookies and provide some scientific support to their traditional use. Obesity and the onset of diabetes are two closely liked medical difficulties prevalent globally. Postprandial hyperglycemia is one of the earliest abnormalities of glucose homeostasis associated with type 2 diabetes. Postprandial glucose levels can be regulated through α-amylase, α-glucosidase and glycemic index inhibition. Me-dicinal plants constitute an important source of potential therapeutic agents for Type 2 Diabetes Mellitus. One vital therapeutic approach is the use of agents that can decrease postprandial hyperglycemia by inhibiting carbohydrate di-gesting enzymes resulting in a delay of carbohydrate digestion to absorbable monosaccharide. In this research work, we intended to evaluate the α-amylase α-glucosidase inhibitory activities and glycemic index of some extracts of Phyllantus amarus cookies to clarify its traditional use as antidiabetic treatment. Obtained results of the enzyme inhibition activity, found in a dose-dependent manner constitute the first report for this plant. Further, in vitro researches are required to confirm the present results, isolate and determine active substances and antioxidants components contained in the extract of this plant may be re-sponsible for improvements in health conditions by regulating digestives en-zymes inhibitory activities. In vitro studies are necessary to recognize a poten-tial substance for clinical use in the therapy of diabetes and related disorders, so it is desirable to optimize secondary metabolite production and purification of compounds for the pharmaceutical applications. The present study confirms the traditional use of P. amarus to treat diabetes mellitus.
