Author(s): Tamuna Gvianishvili*, Liana Gogiashvili*, Maguli Chkhobadze, Elene Nikobadze, Zurab Tsagareli and Tinatin Kvachadze
Oncocytic cell neoplasia makes a challenge in diagnosis and treatment, because It’s known that oncocytic cell neoplasms have unique molecular mechanisms. Oncocytic cells can be seen in many types of thyroid disease, including benign tumors, low-risk neoplasms, and malignant neoplasms. Thyroid tumors are classified as oncocytic cell neoplasms when more than 75% of the tumor consists of oncocytic cells. We studied oncocytic cells in cases of Hashimoto’s thyroiditis and Papillary Thyroid Carcinoma with different markers (Protein S-100, CD56, p63 and Cyclin D1). Based on the obtained results, we can conclude that these markers have different expression in different diseases and is more accurate for oncocytic cell carcinoma than for PTC. However, each marker has different sensitivity and specificity among the diseases studied.
Oncocytic cell lesions are still diagnostic debated in pathology. It is controversial as to the origin of the cell and even which cells in particular may be designated as such still challenge. In 1898 Max Askanazy, a Swiss-German pathologist, were first described “Hürthle cells”, although the name was ascribed to Karl Hürthle, a German pathologist who identified parafollicular “C” cells in 1894 [1].
The new WHO classification has replaced “Hürthle cell” with “oncocytic cell” [2]. Carcinoma of these cells was considered as subtype of follicular thyroid carcinoma. In 2017 it was classified as a distinct tumor type due to its different genetic expression profile, pathologic characteristics, and clinical behavior [3,4]. Oncocytic carcinoma represents about 4% of all differentiated thyroid carcinomas. It’s described more frequently in females and generally diagnosed after the age of 40 [5-8].
Oncocytic cell neoplasms have unique molecular mechanisms, which involve cellular signaling pathways and mitochondriarelated DNA. A polymorphism in the mitochondrial DNA integrity maintainer ATPase 6 gene is believed to play a role in the pathogenesis of oncocytic cell tumors. Oncocytic cell tumors characteristically have been found to have an increased prevalence of mitochondrial DNA common deletions [5,9]. These molecular mechanisms distinguish the oncocytic carcinoma genetic profile from papillary thyroid carcinoma and follicular thyroid carcinoma. Oncocytic cell neoplasms originate in thyroid follicles from follicular cells and are characterized by the presence of oncocytic cells. In generally, these cells are oxyphilic (eosinophilic) cells with round or oval nuclei with prominent typically centrally located nucleolus, and small densely packed mitochondria giving a granular appearance to the empty cytoplasm [9-11].
Oncocytic cells can be seen in other types of thyroid disorders, including non-malignant conditions to a varying degree. Thyroid tumors are classified as oncocytic cell neoplasms when more than 75% of the tumor consists of oncocytic cells [12-14].
Oncocytic cell in thyroid aspirates is seen in variety of lesions such as Hashimoto’s thyroiditis, multinodular goitre, oncocytic cell neoplasms (adenoma/carcinoma), head and neck irradiation, systemic chemotherapy, oncocytic variant of medullary, papillary carcinoma and long-standing Graves disease [14,15].
As we mentioned, oncocytic cells are seen in a variety of nonneoplastic and neoplastic thyroid gland lesions. In thyroid aspirate number and morphology of oncocytic cell differ. For example, thyroid aspirate in focal nodular oncocytic cell hyperplasia in Hashimoto’s thyroiditis comprise of oncocytic cells and sometimes mimics oncocytic cell neoplasia [16,17].
Oncocytic cell neoplasia makes a challenge in both diagnosis and treatment, have unique molecular mechanisms of pathogenesis among follicular-derived thyroid tumors (FDTT), which predict nonprognostic biological behavior of oncocytic cell neoplasia [11].
The research database is a comprehensive treatment for total thyroiditis from both sexes, operational material obtained after total thyroidectomy, unilateral lobectomy and partial resection of the thyroid gland. Retrospective material is also used. Present study was reviewed and deemed exempt from written informed consent by the Ethics committee and Board of medical sciences at Tbilisi State University based on Helsinki-ethical principles declaration for medical research [18].
The material is the tissue samples of the thyroid gland in the operative path. 75% of patients have been treated with lobectomy, totally or partial thyroidectomy. Material received Surgical Card of Tbilisi Clinics and National Center for Interventional Medicine of West Georgia. The total number of patients is 36, of which 22 are Hashimoto’s thyroiditis (HT) and 14 are Papillary Thyroid Carcinoma (PTC).
Tissue samples of thyroid gland, obtained by operated 10% of the formalized buffer solution, are then stained with H&E. After the selection, the material was prepared for immunohystochemical research.
Because in this study, we aimed to analyze the most commonly used immunohistochemical stain for oncocytic cell - protein S-100, adding the new ones Cyclin D1, CD56 and p63, because all three of them represent markers of cells developed from the neural crest, tissue samples were studied using the following antibodies:
1. Protein S-100 (RTU-S100p Polyclone Antibodies) (Biogenex)
2. CD56 (clone, CD564, Leica, UK)
3. p63 (clone 7JUL, Leica, UK)
4. Cyclin D1 (clone D-6, Dako)
Sections were fixed on poly-L-lysine-coated glass slides and prepared as follows: 1) deparaffinization, rehydration and incubation for 20 minutes in 3% H2O2; 2) Immersion in phosphate-buffered saline (PBS) for 20 min; 3) Antigen retrieval in the microwave (600 W) for 20 min, followed by cooling in citrate buffer (0.01 m, pH 6.0). Specimens were incubated with the primary antibodies for 1 hour at room temperature. Then was washed three times with PBS at room temperature. Hematoxyline is used to power the nuclei. All procedures were carried out in compliance with antibodies manufacturer’s protocols (BioGenex, USA; Novocastra, UK).
According our data, the oncocytic cell is characterized cytologically as a large cell with abundant eosinophilic, granular cytoplasm, and a large hyperchromatic nucleus with a prominent nucleolus (fig.1a, b).
It’s interesting, that the results of oncocytic cell carcinoma histopathological and immunohistochemical analysis was shown absence of S-100 protein expression and negative receptor activity against TSH in same tissue area, as our other studies also confirm [19]. And the other hand, current cases demonstrate abundant eosinophilic cytoplasm, expressing the S100 protein (fig. 2 a), in condition positive TSH.
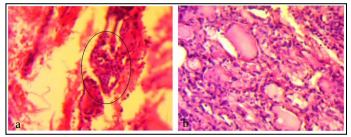
Figure 1: H&E. a) Oncocytic cell neoplasia, X160; b) Hashimoto’s Thyroiditis, X200.
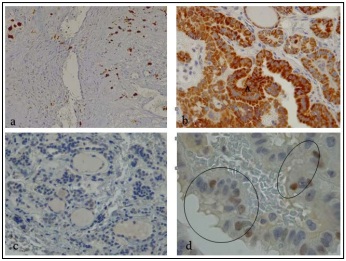
Figure 2: a. PTC, S-100 proteinin oncocytic cell. X200. b. PTC, Cyclin D1 demonstrates diffuse high reactivity in the cytoplasm of follicular (A) cells. X400. c. HT. Protein p63 negative reaction in follicular cells. X400. d. PTC, p63 moderately positive to the apoptotic cells nuclei. X1000. Immunoperoxidase reaction.
Based on these results, we can conclude close relationship between TSH activity and oncocytic cell receptor status.
Protein S-100 staining was also studied in Papillary Thyroid Carcinoma (PTC), and Hashimoto’s thyroiditis (HT). All carcinomas strongly exhibited cytoplasm and nuclear staining for S-100. All cases of Hashimoto’s thyroiditis displayed intense staining in oncocytic cells for S-100, with weak to negative staining in nonoxyphilic follicular epithelium (fig.2 a).
The histologic results suggest including oncocytic cell lesions in the differential diagnosis of protein S-100 positive tumors. We also assessed expression of antibodies CD56 and p63 protein in PTC and HT. All cases were evaluated by immunohistochemistry for the expression of the above mentioned markers. The markers’ patterns and intensities of staining were scored. For example, positive expression of the markers equal or >10% of the follicular epithelium within the tumor or transformed cells was considered positive. An expression of <10% was considered to be negative.
Immunohistochemical images evaluate oncocytic cell with weekly expression of CD56 in cytoplasm, as described in other studies [20,21] in PTC and HT parenchyma; while Cyclin D1 activity revealed strong expression. In oncocytic cell carcinoma all these activity were negative.
During Hashimoto’s thyroiditis CD56 negative or weak positive reaction has been registrated as presence of malignant potential classified as Follicular Epithelial Dysplasia (FED) [19]. CD56 receptor-positive area, in case of PTC, found only in the colloid.
As for p63, in case of papillary thyroid carcinoma tumor tissue reaction in p63 protein was negative and moderately positive to the apoptotic cells nuclei. In contrast, to HT and oncocytic carcinoma, where was fully negative.
As our experience has shown, oncocytic cells present in lesions across the spectrum of thyroid disease. Biological behavior of oncocytic carcinoma is not well understood, newly classified, that why each cases of oncocytic carcinoma lesions make some diagnostic dilemma of thyroid pathology, especially involving in Hashimoto’s Thyroiditis: when a tissue specimen contains oncocytic cells, we must be careful not to miss a malignant transformation.
We concluded that a diagnostic panel, consisted of CD56, S-100, Cyclin D1 and p63, is more accurate for oncocytic cell carcinoma as for PTC. P63 is a specific but less sensitive marker for oncocytic carcinoma than Cyclin D1. CD56 is more specific and sensitive marker than P63, however it is a negative rather than a positive marker for oncocytic carcinoma. As for the protein S-100, it revealed the processes involved in the proliferation of oncocytic cells.
A characteristic structural and immunohistochemical feature of Hashimoto’s Thyroiditis is an increase in the population of oncocytic cells in the parafollicular domain with high nuclear expression of protein S-100. Especially, in adenomatous and infiltrative foci, it determines the dysplasia of the thyroid parenchyma and the architectonics disorganization.
The pattern, distribution and intensity of protein S-100 expression in hyperthyroidism are background and suggest low metabolic activity of specific structures and target cells, such as oncocytic cells compared with classical thyrocytes.
The Author declare that this research work has been conducted in the absence of any commercial or financial relationships, that could be construed as a potential conflict of interest.
