Author(s): Nahla Omer* and Hongyao Xu
Hybrid smart materials, frame both inorganic and organic components, and their contribution in the designing of specific modern technologies are receiving more attention and interest due to their better improved solution process, controllable synthesis, and tunable chemical properties. However, the focus problem of compatibility, solubility and aggregation is to be solved urgently, which inevitably limits them widely application. To this end, an inorganic–organic hybrid material with wide-band absorption is designed and prepared, that incorporates octavinyl-polyhedral oligomeric silsesquioxane (OV-POSS)with amine-containing and cyan uric chloride (CC) to get our novel system OV-POSS-Amine-CC our structure, composition, and properties were characterized and evaluated, the system was prepared as anew path to synthesis novel enhanced hybrid to applied in many application.
In the last contract hybrid materials were progressing on a large scale with a lot of realized economic applications. The increase in the publications and patent applications from the 2005 until the year 2016 is huge .So the early interest in hybrid organic in organic optical based on carbon-silicon networks, many recent efforts have focused on the design of functional hybrid materials which harness the chemical activity of their components. In this field the stakes are high and scientists aim at producing structural materials with properties between those of inorganic and organic hybrid. But the expectations go beyond mechanical strength, thermal and chemical stability; these new materials are also sought for improved optical and electrical properties, luminescence, ionic conductivity, and selectivity, as well as chemical or biochemical activity [1]. Nowadays, in response to the challenge of energy crisis, the immediate imperative is to alternative renewable sources to reduce the emission of carbon dioxide and other harmful substances to our environment, as well as the provision of clean power, solar energy is one of the best promising clean energy to overcome these problems [2].And also the design of fluorescent chemo-sensor with high selectivity and high sensitivity to the detection of various metal ions, have gained remarkable importance in medical and biological applications[3-7],but thesis materials which applied in tow these field have suffering from more problems aggregation effect, compatibility and thermal stability that limits their applications, therefore, how to design well dye-molecular structure; prevent all the problems of the material suffering of it is a big challenge. We select a hybrid material to solve all these problems by OV-POSS, are a class of inorganic compound with well-defined cube-octameric structure with silicalike core surrounded by eight organic corner groups, which make OV-POSS excellent platforms for disbanding of the aggregated platelets., which fabricate our system and enhances the thermal optical limiting, poor compatibility, disaggregation and dielectric properties of organic dyes significantly [8].One of these breeding and promising material connected with the OV-POSS advanced amide due to stability over wide pH intervals (pH 3-11), as well as simple and economical[9],also cyan uric chloride (CC) derivatives have been studied for decade, especially its amino derivatives which advantages of simplicity, high efficiency and excellent selectivity[10].It is an essential organic intermediate of which three chlorine can be replaced by -NH 2 -OH, -SH (or), and -NHR step by step with high yield based on simple thermodynamic control [11]. CC is commercially available and a very inexpensive reagent, which makes it in these days very attractive in applications. In this study, a novel wide-band absorption OV-POSS-Amine-CC is synthesized (Scheme 1).
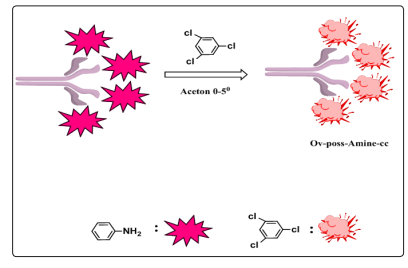
Scheme 1: synthesis road OV-POSS-Amine-CC
Octavinyl-polyhedral oligomeric silsesquioxane(OV-POSS) (AR) was purchased from Shenyang Meixi chemical company,Ltd. Palladium acetate (AR) other chemicals were of analytical grade and obtained from Shanghai chemical reagent company. All chemical reagents were used directly without any further purification.
FT-IR spectra have performed on a Perkin Elmer Model 882 infrared spectrometer in the range of 4000 to 500 cm-1 by mixing each sample with KBr. 1 H NMR spectra were recorded using a Bruker AMX-500 spectrometer operating at 400 MHz, with tetramethylsilane (TMS) as the reference and CDCl3assolvent. The absorbance as a function of wavelength was measured using a Lambda 35 UV-Vis spectrophotometer with 1 cm square quartz cell. The fluorescence spectra were obtained on a PerkinElmer LS 55spectrometer. The electro spray ionization mass spectra were determined by a LCQ Fleet spectrometer (Thermo Fisher).Scans were collected at a resolution of 1 cm-1 scanning from 4000 to 500 cm-1.
Was synthesized by Heck reaction in our previous publication [3].0.8g of hybrid synthesis was added in 50 ml reaction tube with 0.05g 4-bromoaniline 0.022g palladium acetate and 0.01g triphenlphosphine stirred under nitrogen .10 ml Dimethyl formamide DMF and 3ml triethyl amine was added and reacted at room temperature for 12 h then the reaction continued at 100°C for 48h cooled and then filtered through. The solution was rotary evaporated. After dray dissolved with a small amount of THF and precipitated in 200 hexane, this procedure was repeated for 3 times.IR (KBr), υ (cm-1):1106(-si-o), NH 2 (1614), 3440(C-H), 1400-1687(C=C), 1 HNMR in D2O:4.6, 3.4(NH2 ),6.3-7.2(C=C). H1 NMR (400 MHz, ) ? 7.87 (s), 7.87 (s), 7.81 (d, J = 50.0 Hz), 8.14 - 7.65 (m), 8.14 - 7.26 (m), 7.17 (d, J = 17.8 Hz), 5.55 (s), 3.74 (d, J = 4.4 Hz), 3.50 (s), 3.49 - 3.08 (m), 3.08 - 1.44 (m), 1.30 - 1.23 (m), 0.90 (s), 0.09 (s).
Was synthesized by following the method hybridOV-POSS-Amine 0.2g was added slowly to cyan uric chloride, 1.845g in acetone 35 ml with constant stirring for 4h at 0°C. Sodium carbonate sol 10% was added to neutralize HCl evolved during the reaction. Finally the content were poured in to crushed ice the solid separated was filter washed with water dried, and recrystallized from ethanol many times. IR (KBr), υ (cm-1): 1465,(C-N),870(C-Cl),1 H NMR (400 MHz, CDCl3) ? 7.27 (t, J = 7.5 Hz, 88H), 6.58 (d, J = 8.5 Hz, 84H), 5.15 - 1.75 (m, 98H), 1.91 (s, 3H), 1.27 (t, J = 7.1 Hz, 1H), 0.13 - 0.01 (m, 17H).
The UV absorption in Figure 1 attributable to conjugation of hybrid OV-POSS-Amine at 410 nm.The absorption at 510 nm, and 760 nm for Semi-Squaraine (SSQ) and Squaraine (SQ)before conjugated with amine in our work [2], after conjugated with amine; it became 545 nm and 765 nm. The absorbance located in275nm for hybrid OV-POSS-Amine-CC. As can be seen the progress of the reaction by absorption spectroscopy. This indicates that the reaction good conjugated and successful, our system was water-soluble.

Figure 1: UV-visabsorbancespectra hybrid OV-POSS-Amine, hybridOV-POSS-Amine-CC
The 1 HNMR of hybrid OV-POSS-Amine, hybrid OV-POSSAmine-CC. Figure 2 show characteristic bands the amine protonsNH 2 at 4.7 ppm a and the proton of aniline ring at 6.3-7.2 ppmb for hybrid OV-POSS-Amine-CCthe amino group changed to amide group at10 ppm athe proton of aniline ring at 7.1-8.2 ppm b and the proton of cyan uric chloride c at 2.5ppm.These results confirmed that the successful incorporation of our system and get hybrid OV-POSS-Amine-CC.
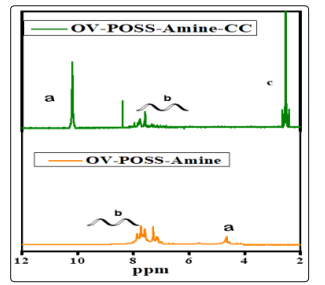
Figure 2: H1NMR spectra of hybrid OV-POSS-Amine, hybrid OV-POSS-Amine-CC
FT-IR spectrum showed new peaks of hybrid OV-POSS-Amine, hybrid OV-POSS-Amine-CC shows in Figure 3 The IR spectrums of OV-POSS include an intense characteristic Si-O-Si stretching at 1106 cm- 1. Moreover, and appearance of a new broad peak near the 1580-1650 cm-1confirmed to the groupamineNH2 . Also, absorption appeared in the FTIR strong stretching vibration of the group C-N cm-1 at 1465cm-1, at 870cm-1for bending of C-Cl and the amide group at 3000 to 3500 cm-1which indicates that synthesis was successful.
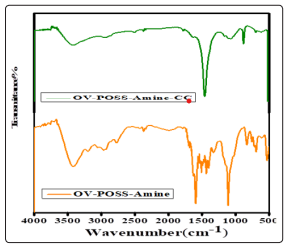
Figure 3: IR spectra of hybrid OV-POSS-Amine, hybrid OVPOSS-Amine-CC
The morphology of hybrid OV-POSS-Aminewas observed in Figure 4 (a), more porous surface shape was observed. And the morphology of the hybrid OV-POSS-Amine-CCwas show in Figure 4 (B) stacked lamellar structure. So that OV-POSS treat the interfacial strength and the interfacial compatibility. This might be refer to the lubricating effects of OV-POSS particles, which made easier the molecular movement of the structure, detect that the dispersed phase particles size was reduced in the presence of OVPOSS. In the thin film of with hybrid OV-POSS-Amine-CCshows observed to be uniformly covered with the OV-POSS domains, where good compatibility is achieved in this morphology. And also the prorogated aminesgroups make it very soluble in water. Furthermore, taking into account its small particle size and good disparity in aqueous solution.

Figure 4: FE-SEMof (a) hybrid-POSS-Amine (b) hybrid OVPOSS-Amine-CC
The traditional materials were suffering ofaggregation effectand compatibility when applying in solar cell and biological imaging, sowe have highlighted recent progress towards the development hybrid system OV-POSS-Amine-CC designed and prepared successfully. Our work has opened new road for the novelty of new synthesis
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (Grant Nos. 21471030, 21671037, and 21771036).
