Author(s): Amrita Anand
Multiple myeloma (MM) is a plasma cell neoplasm accounting for 1% of all malignancies. It is characterized by a monoclonal malignant proliferation of plasma cells accompanied by an increase in M-protein. Plasmablastic myeloma represents 5-15% of the cases of multiple myeloma. This morphology of a MM is an independent predictor of poor survival. Plasmablastic myeloma tends to have worse outcomes than other plasma cell dyscrasias. The median survival of these patients is around 1.9 years. Much of our knowledge on plasmablastic variant of MM is dependent on case reports and case series. Hence, an early identification of this aggressive variant of multiple myeloma and its differentiation from hematological malignancy like plasmablastic lymphoma is necessary for optimal patient management.
The patient was a 70-year-old male who presented in the orthopedic OPD with complaint of easy fatiguability and lower backache for 6 months. The patient gave no history of fever or trauma. The patient’s past medical history was insignificant. General physical examination showed mild facial puffiness and patient was found to be hypertensive with a recorded blood pressure of 140/90mm Hg. There was no lymphadenopathy or organomegaly.
Laboratory investigations done previously revealed anemia (hemoglobin 8.2 g/dL), thrombocytopenia (platelet count 1 lakh/ mm3 ), Total leucocyte count (9200/mm3 ), Differential count N70 L20 E07 M01, Serum total protein 6.5g/dl (normal 6.0-8.3g/dl), Albumin 3.5g/dl (normal 3.4-5.4g/dl), hypoglobulinemia 1.54g/dl (normal 2-3.5 g/dl). The urine output was decreased to 250ml/day (normal 800-2000ml/day). The serum protein and urine electrophoresis were within normal limits, and a mononoclonal band was absent.
Urine was negative for Bence Jones proteins. The patient was seronegative for viral markers including human immunodeficiency virus (HIV), hepatitis B surface antigen, and hepatitis C virus (HCV). Radiological investigations were also unremarkable. There were no lytic lesions seen on X-ray and MRI. There was absence of any pathological fractures. A repeat complete blood count (CBC) was done at our center and it showed few atypical lymphoid cells. A provisional clinical diagnosis of Non Hodgkin Lymphoma or Multiple Myeloma was considered and investigations were started.
A repeat CBC with peripheral smear along with bone marrow aspirates were sent in our hematology lab. Wright-Giemsa stained peripheral blood smears (Figure 1,2) showed the presence of 8% blasts. The red blood cells showed mild anisocytosis with presence of normocytic normochromic cells and few polychromatophils. The white blood cells showed a mild shift to left. DLC: Blast 08 Myelocyte 01 Metamyelocyte 03 stab 03 Neutrophil 68 Lymphocyte 14 Eosinophil 01. The bone marrow aspirate smears (Figure 3,4) showed the presence of 74% blasts. These blasts were 2.5 to 3 times the size of small mature lymphocyte, having scant to moderate amount of agranular cytoplasm.
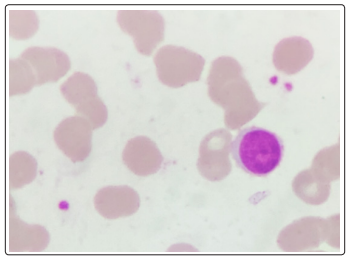
Figure 1: Peripheral smear Wright - Giemsa, 1000x, Blast
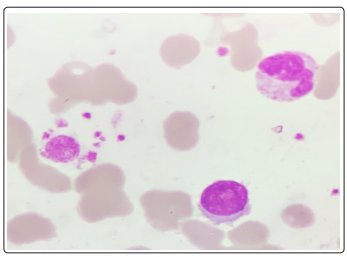
Figure 2: Peripheral smear Wright - Giemsa, 1000x, Blast, neutrophil and platelets
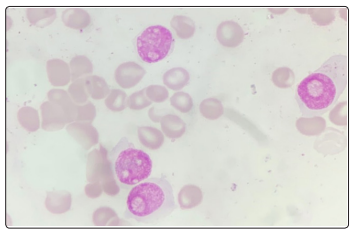
Figure 3: Bone Marrow Aspirate: Wright - Giemsa, 1000x, Blasts showing plasmablastic morphology
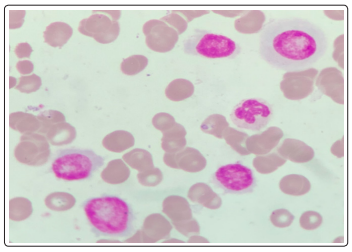
Figure 4: Bone Marrow Aspirate: Wright - Giemsa, 1000x, Blasts showing plasmablastic morphology
The nuclei were central to eccentrically placed, having a high nucleo-cytoplasmic ratio, opened up chromatin and a prominent nucleoli. There was also the presence of many immature and mature plasma cells. Myelogram was given as Blasts74 Plasma cells04 nRBC06 Myeloid04 Stab03 Neutrophil06 Lymphocte03. The differential diagnoses kept at this stage were plasma cell myeloma, plasma cell lymphoma, undifferentiated leukemia, highgrade B-cell lymphoma. A flowcytometric analysis (Figure 5-14) was undertaken on Beckman coulter FC 500, to identify lineage specificity and typify the neoplasm. No population was identified on CD45 vs Side Scatter, in fact a population was identified in the the CD45 negative region, questioning our diagnosis of a leukemia.
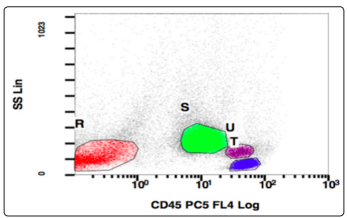
Figure 5: CD45 v/s SSC, Population of interest: R (CD45 negative)

Figure 6: CD10 v/s CD19, Population gated: R (CD10, CD19 negative)
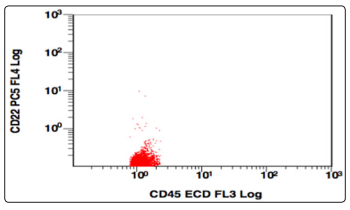
Figure 7: CD45 v/s CD22, Population gated: R (CD45,CD22 negative)
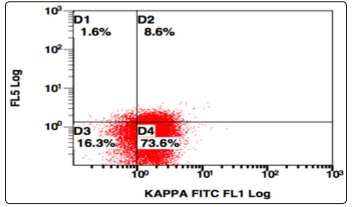
Figure 8: SSC v/s Kappa, Population gated: R (Kappa positive)

Figure 9: SSC v/s Lambda, Population gated: R (Lambda negative)
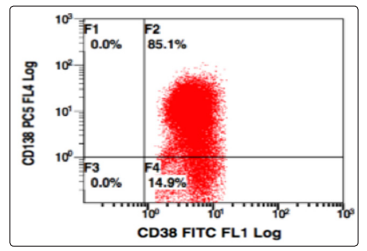
Figure 10: CD138 v/s CD38, Population gated: R (positivemoderate expression of both CD38, CD138)
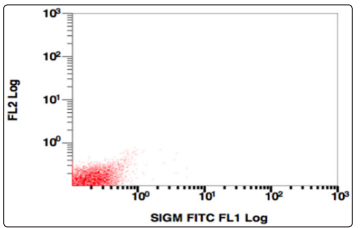
Figure 11: SSC v/s surface immunogloblin, Population gated: R (sIgM negative)
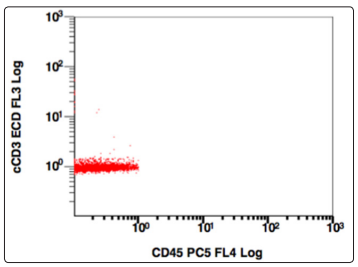
Figure 12: CD45 v/s cCD3, Population gated: R (cCD3 negative)
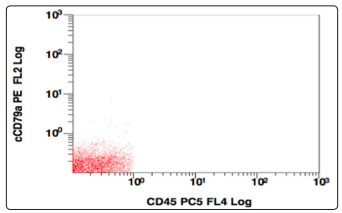
Figure 13: CD45 v/s CD79a, Population gated: R (CD79a negative)
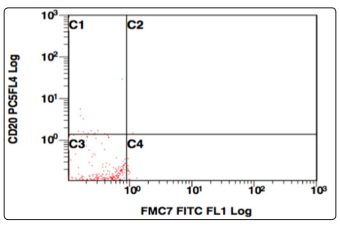
Figure 14: CD20 v/s FMC7, Population gated: R (CD20, FMC7 negative)
Further the population gated in the negative window was showing no positivity for any B-cell (CD19,10,20,22) or T cell markers (CD3,CD5,CD7). More markers were added in the panel and gating was done, this time using CD38 v/s side scatter. A huge CD38 positive population was identified, and on further analysis, this population was positive for CD138, and showed a kappa restriction. This population was negative for sIgM, CD23, CD79a, MPO, Tdt. On combining the morphological and flowcytometric findings, a diagnosis of Plasma cell Myeloma was given and the patient was started on chemotherapy.
Plasmablastic multiple myeloma is a morphologic variant of multiple myeloma containing ≥2% plasmablasts [1]. Plasmablasts are the most immature form of plasma cells and this morphological variant represents 5-15% of the cases of multiple myeloma [2]. Morphologically, a Plasmablastic Myeloma is characterized by the presence of a cell with the nucleus having a fine reticular chromatin pattern (no or minimal chromatin clumping); large nucleus (estimated to be greater than 10 μm), prominent large nucleolus (estimated to be greater than 2 μm); scant cytoplasm (must have no or very little hof region), and less abundant cytoplasm (less than one-half of the nuclear area) [3]. Plasmablastic morphology confers a very poor prognosis and survival even after an autologous stem cell transplantation is also poor [4].
A Plasmablastic Myeloma must be differentiated from lymphoid neoplasms with immunoblastic, or plasmacytoid features. These include plasmablastic lymphoma diffuse large B-cell lymphoma (DLBCL), ALK-positive large B-cell lymphoma [5]. The closest differential for a plasmablastic myeloma is plasmablastic lymphoma. Both the malignancies show a characteristic morphiological and immunophenotypical overlap [6]. Plasmablastic lymphoma is an aggressive B-cell malignancy with neoplastic cells, resembling plasmablasts [7]. These cells express the plasma cell markers likeCD38, CD138 making the diagnosis even more challenging [7]. It is clinically important and relevant to differentiate the two entities.
The treatment for both the malignancies as well as prognosis is highly different and it requires a meticulous clinical, morphological and flowcytometric analysis to differentiate between the two [8]. Colomo et al in their study concluded that CD56 was a specific for plasma cell myelomas [9]. Peroxiredoxin I, another marker which is expressed in a differentiated plasma cell, and not in mature B-lymphocytes, can be used to identify for plasma cell neoplasms [10]. A DLBCL shows positive immunostaining for B-cell markers like CD20 and CD79a, very infrequently expressed CD138 [11]. An ALK-positive large B-cell lymphoma usually lacks the expression of B-cell and plasmacytic markers like CD20, CD79a, and CD138, and are usually positive for cytoplasmic IgA [12]. These cells can show pseudopodial cytoplasmic projections resembling a plasma cell [12].
The Eastern Cooperative Oncology Group (ECOG) trial E9486 reviewed 37 plasmablastic cases and emphasised the role of RAS mutations and an increased serum levels of soluble IL-6R in a Plasmablastic morphology [3]. The trial concluded that these cases tend to have a more advanced and aggressive disease manifested by more frequent anemia, renal insufficiency, hypercalcemia, a higher PCLI, high beta-2 microglobulin level, and a lower albumin level. They hypothised that the aggressive behavior of plasmablasts and PB MM may in part reflect an increased IL-6 responsiveness associated with Ras mutation [3]. PBM patients should be considered as having high-risk myeloma particularly forms with extramedullary disease and treated accordingly [13].
Conclusion
Plasmablastic myeloma represents a discrete entity and is an independent prognostic factor predicting shorter EFS and OS [3]. Plasmablastic myeloma is a tricky diagnosis and its differential diagnosis are difficult to separate, if only the cytomorphological features are analyzed. It is therefore suggested that cytomorphology combined with flowcytometry/immunohistochemical analysis and a complete clinical work up is required for making the correct diagnosis.
