Author(s): R C Jagessar
Porphyrins are terapyrrolic compounds that are usually synthesized by the condensation of aromatic aldehyde with pyrrole or the reaction of dipyrromethene with an aromatic aldehyde followed by oxidation with p-chloranil. Because of its highly conjugated nature and rich 22 p electron system, excellent chemical and physical properties, they have been used as versatile building blocks for the synthesis of nanomaterials with varying applicability, extreme molar absorption to near-infrared spectrum from the visible region, supreme oxygen quantum yields with singlet state, and chemical flexibility. Functionalized porphyrin-based nanocomposites offers various tunability modes in terms of optical, electronic, and magnetic properties and provide an efficient sensing platform to detect various analytes in the field of environment, health, and food. Porphyrin carbon nanocomposites can be used as nanosensors for the detection of toxic compounds and volatile organic compounds (VOCs) generated during the deterioration of food. Porphyrins have demonstrated great potentials in visible-light photocatalytic applications, because of their electrical, optical and catalytic properties. This has found diverse application in the synthesis of selective nanomaterials with porphyrin like properties. The nanoparticles of porphyrin and its derivatives have been developed for drug delivery systems which are one of the popular fields in pharmaceutical chemistry. Porphyrin nanomaterials condensing to different drug delivery variables have been utilized to enhance delivery features due to the properties that allow immune tolerance, specific targeting, better hydrophilicity, and lengthy tissue lifetime.
Porphyrins are a class of terapyrrolic macrocyclic compounds. Structurally, a porphyrin ring consists of four pyrrole rings joined by four methine bridges at the -carbon of the pyrrole molecule. The structure of the simplest porphyrin, porphine is shown in Figure. 1. They are a class of heterocyclic molecules. They have a total of 26 electrons of which 18 are in a planar, continuous conjugated macrocycle. Evidences for aromaticity are confirmed by measurements of their heats of combustion and the planarity of the nucleus. Both free base and metalloporhyrins are planar in nature. They can be functionalized to form extended superstructures such as “picket fence” porphyrins. Free base
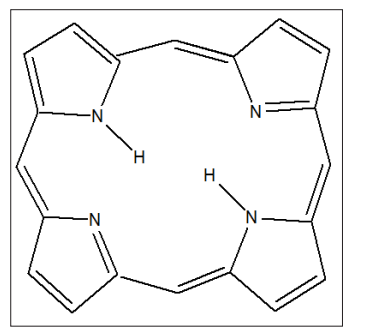
Figure 1: Porphine, the Simplest Porphyrin
porphyrins are those without a metal in the centre. The four nitrogens in the centre can complex a range of transition metals to form a wide range of metalloporhyins, each of which has its unique metalloporphyrin characteristics [1-2]. Both “free base and metalloporhyrins” have rich photo fluorescence electronic properties. They have also found increasing application in catalysis and photocatalysis. Porphyrins because of their rich photosensing activities have been used in photodynamic therapy (PDT) to kill cancer cells. Porphyrins are usually synthesized via the traditional method involving the condensation reaction of pyrrole and benzaldehyde or the reaction of the reaction of dipyrromethene with an aromatic aldehyde followed by oxidation with p-chloranil. In recent years, porphyrins have been finding increasing application in nanotechnology. Thus, this paper reviews the recent advances in porphyrins based nanomaterials.
Porphyrins used as a photosensitiser to treat cancer via Photodynamic Dynamic Therapy (PDT) have been limited to the fact that porphyrins tend to form agglomerates or aggregates in aqueous solution which leads to their low bioavailability [3- 4]. Since, the human biological system consists mostly of water, it means porphyrins used in PDT is limited by this property. However, porphyrins based nanomedicines has brought a new dimension to porphyrin nanomedicines. These include
Porphyrin based nanomaterials as nonomedicines include: Porphyrin based liposome (Porphysome), Porphyrin based micelles, porphyrin based polymeric nanoparticles, Porphyrin-Peptide based nanoparticles and porphyrin based small molecule nanomedicines [5].
The synthesis and usage of Porphyrin based nanostructures for sensing applications have been reported6. They have been used for solid state sensors for volatiles and metal ion recognition and detection. In this regards, Porphyrins Nanotubes and Amphiphilic Porphyrin aggregates have been explored as chemical sensors. The presence of the inner cavity of porphyrin nanotubes can promote endohedral inclusion of different guests and analytes, thus promoting their sensing proficiency [6].
Porphyrins and their derivatives are abundant in nature and play an important role in plant photosynthesis. To mimic the photosynthetic reactions in plants, researchers have developed numerous porphyrin-based nanomaterials to convert light into chemical energy and porous porphyrin-based nanomaterials attract great interest in photocatalysis owing to their high surface areas, designable structure, and favorable photocatalytic behavior [7].
Porphyrins as nanoreactors such as covalent–organic frameworks and metal–organic frameworks have been used in the carbon dioxide capture and conversion into value added product. Porphyrin can achieve this feat a result of the presence of the basic pyrrole structure that contains a macrocyclic cavity and large aromatic rings, which facilitate strong interactions with CO2 [8]. The photoconductivity of nanorods self-assembled from meso- tetrakis (4-sulfonatophenyl) porphine has been reported. The nanorods insulates in the dark. Upon illumination with 488 nm light, the nanorods become photoconductive, exhibiting a rapid turn on/off (<100 ms) of the current when the light is turned on/off. This photoconductivity grows over hundreds of seconds with light exposure and decays slowly when the light is off. The nanorods can be trained via an applied bias to exhibit a short-circuit photocurrent (with corresponding open-circuit photovoltage) that flows in the direction opposite that of the training bias. A qualitative model is proposed, in which conduction occurs through the tightly coupled LUMOs of close-packed porphyrin molecules [9] .
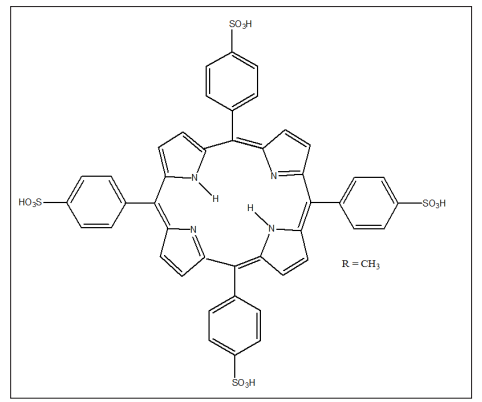
Figure 2: Meso-Tetrakis(4-Sulfonatophenyl)Porphine
Porphyrin nanorods produced from the sodium salt of tetrakis (4-sulfonatophenyl) porphyrin, dissolved in acidic aqueous solutions, were deposited onto Au(111) substrates and imaged by atomic force microscopy (AFM) and scanning tunneling microscopy (STM). The AFM and STM images revealed individual rods with a diameters of 25−40 nm and lengths of hundreds of nanometers. For the first time, high resolution STM images of TSPP on Au(111) were obtained and revealed that the rods are composed of disk-like building blocks approximately 6.0 nm in diameter. The UV−visible absorption spectrum of the porphyrin solution phase aggregate was also found to be similar to that of the aggregate deposited on quartz, confirming similar ground and excited electronic state structures of the excitonically coupled chromophores [10].
Nanocrystals have great application in areas of photonics, electronics, and optics. The fabrication of linear chains of Au nanorods with bifurcated junctions of nanorods/nanospheres was achieved via the crosslinking of H-type tetrakis(4-sulfonatophenyl) porphyrin aggregates in solution. Modulation of the plasmon coupling between the Au nanocrystals was demonstrated by varying the porphyrin concentration and the interparticle gap distances. Finite-difference time-domain calculations revealed that the red shift of the plasmon band exhibits a nearly exponential decay with increasing interparticle gap distances, thus giving rise to a “plasmon ruler equation.” The gap distances are in accordance with the experimental observations and further confirm the porphyrin- directed assembly process. The mechanistic interaction between the Au nanorods and porphyrins was investigated by a biological procedure using the dark-field light scattering technique [11] .
On surface synthesis within a Porphyrin Nanoring Template has been noted. On surface synthesis provides a route for the production of 1D and 2D covalently bonded polymeric structures. These reactions are confined to the surface of a substrate and the catalytic properties of the substrate are utilized to initiate the reaction. Here, cyclic porphyrin polymers, nanorings, consisting of 40 porphyrin units with an internal diameter of 13 nm were used to form a template on a Au (III) surface. An on-surface Ullmann type coupling reaction was iniated within the nanoring template. The covalently coupled reaction products were investigated and characterized with scanning tunnelling microscopy [12].
The synthesis of ethyne-linked porphyrin nanorings has been achieved by template-directed Sonogashira coupling. The cyclic hexamer and octamer predicted adopt low symmetry conformations, due to dihedral twists between neighbouring porphyrin units with symmetries of D6h and D8h, respectively, in solution by 1H NMR [13]. The singlet excited states of these nanorings are highly delocalized as revealed by the fluorescence spectra.

Figure 3: Porphyrin based Nanorings and Corresponding Absorption and Emission Spectrum
The conductance of Porphyrin based molecular wires have been shown to increase in length. Molecular nanowires for high electrical conductance are necessary for future molecular scale circuitry. Typically, molecular wires act as tunnel barriers and their conductance usually decays exponentially with length. However, it has been reported that the conductance of fused oligo porphyrin nanowires can be either length independent or increase with length at room temperature. The potential use of these molecular wires as interconnects in future molecular scale circuitry is noted [14].
Self-assembled molecular wires were prepared step-by-step, alternating up to 13 units of zinc-octaethylporphyrin with axially coordinated 4,4′-bipyridine, on highly oriented pyrolytic graphite (HOPG). Molecular resolution imaging and scanning tunneling spectroscopy were used to follow molecules self-assembly in real time during wire fabrication and to measure wires current, respectively. A statistical analysis of hundreds of current–voltage curves was carried out to determine the conductance of individual porphyrin/bipyridine wires. From the conductance dependence on the wires length an ultra low attenuation factor (β = 0.015 ± 0.006 Å–1) was obtained for shorter wires, with a transition in conduction regime occurring at ca. 6.5 nm long wires [15].
Short chains of porphyrin molecules can mediate electron transport over distances as long as 5–10 nm with low attenuation. This means that porphyrin-based molecular wires could be useful in nanoelectronic and photovoltaic devices, but the mechanisms responsible for charge transport in single oligo-porphyrin wires have not yet been established. Based on electrical measurements of single-molecule junctions, we show that the conductance of the oligo-porphyrin wires has a strong dependence on temperature, and a weak dependence on the length of the wire. Although it is widely accepted that such behaviour is a signature of a thermally assisted incoherent (hopping) mechanism, density functional theory calculations and an accompanying analytical model strongly suggest that the observed temperature and length dependence is consistent with phase-coherent tunnelling through the whole molecular junction [16].
The Tuning of the electrical conductance of metalloporhyrin supramolecular wires have been noted in the literature. In this article, the electrical conductivity of M(II)-5,15-diphenylporphyrin (M-DPP) single-molecule junctions (M = Co, Ni, Cu or Zn divalent metal ions), in which the current flows perpendicular to the plane of the porphyrin is contrasted with conventional single molecule junctions, in which the current flows parallel to the long axis or plane of a molecule. Novel STM based conductance measurements, combined with quantum transport calculations demonstrate that current perpendicular to the plane (CPP) junctions have three orders of magnitude higher electrical conductances than their current in plane (CIP) counter parts. This current range from 2.10-2 G0 for Ni-DPP up to 8.10-2 G0 for Zn-DPP [17-18].
Porphyrin, the green pigment of life has been an indispensable building block in many realms. This include: natural and artificial photosynthesis, photodynamic therapies, light harvesting systems, catalysts, chemical sensor technology, etc. Recent advances have seen their usage as porphyrin nanomaterials via porphyrin based molecular wires, porphyrin based molecular sensors, porphyrin nanorods and porphyrin nanorings. All these materials show unique, superior properties compared to conventional monomeric and dimeric porphyrins.
