Author(s): Arathi Anil*, Arun Biju Balakrishnan KV, Maya Rajan Peter and Reshma Suresh
ABSTRACT: Periodontal diseases are common inflammatory diseases that affect the gingiva and tooth supporting apparatus. In the early stages treatment of periodontal disease involves reversibility of gingivitis so that progression to periodontitis is halted. Resolution of inflammation plus the biofilm attached to the tooth surfaces removed through mechanotherapy, gingival health is restored. However, in the later stage of the disease, periodontitis is irreversible and nonresolving in nature. Therefore, it is generally accepted that etiology of periodontal disease is microbial in nature. Disease progression itself is host mediated. Various modifiable and non-modifiable risk factor contribute to development of periodontitis.
Diagnosis represents the backbone of successful periodontal treatment since the entire treatment plan, prognosis, and maintenance directly depend on the quality and precision of periodontal diagnosis. Various modifiable and non-modifiable risk factors also contribute to the etiology and diagnosis of periodontal disease. The use of biomarkers has been introduced for the first time within the new classification of periodontal and peri-implant conditions as a first step towards the adoption of precision medicine concepts in periodontology. But a single biomarker will not be beneficial for finding the entire etiology of the periodontal disease. This review elaborates the unmet diagnostic needs in periodontal diagnostics, the concept of precision periodontics, periodontal biomarkers, and a pipeline for accelerated implementation of the precision medicine approach in periodontal practice [1].
Ideal biomarkers of periodontitis must be able to diagnose the presence of periodontal disease, reflect the severity of the disease monitor the response of the disease to treatment, and predict the prognosis/progress of the disease [2-4].
A biomarker is“a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention” [3]. The great advantage of periodontal marker assessment is the accessibility of diagnostic specimens that can be obtained by low invasive and inexpensive procedures. Hence, dental plaque as a diagnostic specimen for microbiological assessment can be easily collected by means of sub-gingival swab using standardized precut methylcellulose filter strips or using universal endodontic paper of higher caliber (>30) [4,5].
The traditional clinical assessment methods include attachment level, probing depth, bleeding on probing, radiographic assessment of alveolar bone loss, but they neither provide information on the measures of disease activity nor do they identify the individuals who are susceptible to future disease progression as the biologic phenotypes are not reflected properly in the clinical phenotype [6]. Biological phenotypes may then be taken into consideration which will be of help in assessing the burden of microbial and inflammatory load, which further affects the progression of periodontitis [7].
Field of medicine commonly uses oral fluid-based POC diagnostics and lately it is being employed as the potential“chairside” test for determination of oral diseases [8]. New technologies like ‘lab-ona-chip’ and microfluidic devices have emerged as a great hope in managing oral fluids such as saliva and gingival crevicular fluid and they also determine patient’s periodontal disease-risk profile, current disease activity and response to therapeutic interventions. This approach in turn proves to be useful in a chronic infectious disease such as periodontitis in terms of monitoring of episodic nature of this disease and in making clinical decision [9]. The various vehicles used for assessing periodontal disease activity are saliva, serum, GCF but because saliva and GCF are fluids that can be collected with ease and are rich in locally and systemically derived markers of periodontal disease, these hold a great potential for the assessment of patient-specific biomarker in the diagnosis of periodontitis and other systemic diseases [10].
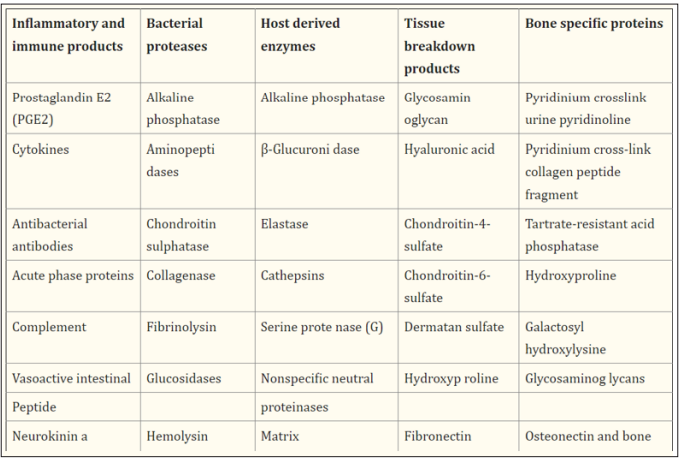
Saliva is an optimal biological fluid to serve as the diagnostic tool for periodontitis. The collection of saliva is safe, noninvasive, and simple, and saliva can be collected repeatedly with minimum discomfort to the patient. Salivary biomarkers of periodontal disease can originate from both bacteria and the host. As periodontitis progresses, gingival inflammation, soft tissue destruction, and bone destruction occur sequentially and release associated proteins or metabolites into the saliva. Therefore, hostderived biomarkers are categorized according to whether they reflect inflammation, soft tissue destruction, or bone destruction.
The biomarkers that satisfy three of the four requirements in at least three separate studies are classified as strong (S) biomarkers. When the number of studies that reported no difference or contradictory results is equal or greater than those with supporting results, the biomarkers are classified as questionable (Q). The remaining biomarkers are classified as potential (P) [2].
These include DNA and proteins. The levels of wellknown pathogenic bacteria, such as Aggregatibacter actinomycetemcomitans, the three red complex species, and several species of the orange complex in saliva were determined by targeting a specific area of the 16S rRNA gene. Among them, only Porphyromonas gingivalis, Prevotella intermedia, and Tannerella forsythia have been proved by multiple studies as strong biomarkers of periodontitis. Recent studies using high-throughput sequencing of the 16S rRNA gene have identified new species/ phylotypes that are associated with periodontitis [11]. Dipeptidyl peptidase IV is a serine protease that cleaves X-Pro dipeptide from the N-terminus of polypeptide chains, thus contributing to collagen degradation [12].
As inflammatory biomarkers in saliva, diverse enzymes (arginase, dipeptidyl peptidase IV, β-glucuronidase, and myeloperoxidase), anti-microbial proteins (lactoferrin and calprotectin), inflammatory cytokines (IL-1β, IL-6, IL-18, IFN-γ, and MIP-1α), and proteins that mediate inflammation (chemerin, CRP, TLR4, soluble CD14, and procalcitonin) have been studied. Particularly, IL-1β, MIP-1α, and arginase are strong biomarkers that correlate with inflammatory parameters of periodontitis, such as the gingival index or BOP. In addition to protein biomarkers, nitric oxide, 8-hydroxydeoxyguanosine, platelet activating factor and fatty acid metabolites (neopterin, docosapentaenoate, linoleate, and arachidonate) have been identified as inflammation-associated biomarkers in saliva [13].
GCF, is a body fluid derived from serum, leukocytes and cells of the periodontium and oral microflora [14]. Its composition is the result of the interplay between the bacterial biofilm and the cells of the periodontal tissues. The++ specific composition of GCF is the biochemical indicator of the locally produced changes in metabolism, thus determining the periodontal status of the individual. Since host response is a critical determinant in periodontal disease pathogenesis, inflammatory mediator levels in the GCF can be used to evaluate ‘risk’: risk for a tooth, or more precisely a site where clinical attachment and alveolar bone may be lost, or risk for an individual to develop periodontal disease [15]. GCF contains a variety of potential markers derived from host and bacteria from supragingival and subgingival plaque thus, offering a wide array of candidate makers for detection of periodontal disease activity.
Although a biomarker as an indicator of a biological process can be used to study the characteristics of physiological or pathological conditions and their respective responses to different factors (such as treatment), biomarkers validated for diagnostic use need to comply with specific diagnostic requests defined in rigorous guidelines for biomarker validation, varying for each biomarker subgroup [16]. In the context of biochemical markers, proteomics is an analytical method that can contribute to the identification of highly specific cytokine panels, and different multiplexing methods enable the assessment of a great range of protein profiles [17]. Finally, the bone level represents the epicenter of the entire dental implant concept, and there is no method more accurate for real-time assessment of ongoing bone processes than the direct measurement of bone metabolism. he advanced“omics” methods that can particularly contribute to the identification of new bone markers are metabolomics, particularly regarding identification of the byproducts of bone destruction for real-time assessment of changes in the bone level over time or in response to treatment. Histopathological studies will contribute to biological definitions of the grading criteria of periodontitis. Finally, the entire process of biomarker validation directlydepends on the quality of the clinical aspect of diagnostic studies. Hence, strict adherence to the clinical diagnostic criteria and case definitions defined in the referent classification of periodontal conditions is the ultimate precondition for accurate validation of periodontal biomarkers. Further, studies aiming to validate surrogate endpoints for the assessment of periodontal treatment outcomes should be first conducted according to guidelines for standard periodontal treatment [16].
Precision periodontics undoubtedly represents the future of highquality periodontal care, so it is of paramount importance that future research studies strictly adhere to the recommendations for the validation of biomarkers in order to accelerate the process of their implementation in routine clinical practice. Moreover, future studies should focus on the development of biomarker assessment protocols applicable in everyday practice, such as point-of-care testing (poct), which is still in the developmental stage in periodontology [18,19].
