Author(s): MT Cabaraban*, G Divinagracia, JC Padernal, VM Ramirez, L Arranguez, JM Semilla, DJM Ombiga, EJ Barcelona and K Paderanga
Cassava pulp (Manihot esculenta Crantz) residue (CPR) is the solid waste generated from the cassava processing industry. In the bench-scale experiments, fast oxidative pyrolysis of CPR was carried out in a fluidized bed reactor, using uncondensed recycled pyrolysis gas as carrier medium to produce charcoal and biocrude. The influence of three gas recycle rates, ranging from 0.60 to 2.3 Sm3 h –1 , on the product yields and characteristics was investigated. Results suggest that an increase in the recycle rate will lead to an increase in biocrude yield and a decrease in the charcoal yield. The product yields of biocrude and charcoal were 15.68 ± 2.08 percent and 24.29 ± 3.31 percent, respectively. The biocrude product was mainly composed of alcohols, phenols, aldehydes, ketones, alkanes, and alkynes. The charcoal obtained has a heating value that is around 85 percent higher than that of the CPR feedstock. It has a high fixed carbon content of around 67 percent, but a low volatile matter content of only around 28 percent. At the pyrolysis temperature of around 500 °C, the uncondensed pyrolysis gas compounds consisted primarily of N2 and C2 H6 , with small amounts of H2 , and higher hydrocarbon compounds. Results suggest the potential for generating biofuel products from the fast oxidative pyrolysis of CPR.
Biomass is widely recognized as a critical renewable source that can supply increasing amounts of biofuels and bioenergy, with the potential to reduce carbon and other greenhouse gas emissions. Biomass feedstocks for biofuels and bioenergy are categorized into first (e.g. edible crops), second (e.g. energy crops, lignocellulosic biomass from agro-waste, organic fraction of municipal solid wastes), third (e.g. macro- and microalgae) and fourth generation (photobiological solar fuels, electrofuels) [1,2].
The technology for the production of biofuels from fourth generation sources is still in its infancy, and further research can significantly improve its energetic and economic feasibility [3- 5]. The economic feasibility of the production of biofuels from third generation biomass feedstocks is still problematic because of major technical challenges, and high energy and capital costs [6-8]. The utilization of first generation biomass feedstocks raised concerns about competing land use, and could lead to increases in global food prices [2,9]. Second generation biomass sources, since these are lignocellulosic nonfood crops and can grow on marginal or degraded lands, address the issues associated with first generation sources [10,11]. Biomass wastes originating from agri-processing industries have been investigated as another alternative source from which to obtain value-added products, without competing with land for food production while mitigating the environmental impacts [12-16].
Fast pyrolysis is the thermochemical decomposition of lignocellulosic biomass in an oxygen-deprived atmosphere at temperatures between 450 °C and 650 °C for a short vapour residence time to produce biocrude (or bio-oil), charcoal and noncondensable gases [17]. This process has received a great deal of attention in recent years [12,17-22]. However, maintaining an oxygen-deprived environment necessitates separating nitrogen from air or using the more expensive noble gases (helium or argon) as fluidization gases (carrier gases), imposing additional costs to the process.
Some attempts have been done to pyrolyze biomass using different fluidization gases which have potential of reducing the operating cost. For example, Zhang et al. used various main pyrolysis gas components, namely N2 , CO2 , CO, CH4 and H2 , as carrier gases for the fast pyrolysis of corn cobs in a fluidized bed reactor [23]. Guizani et al. performed biomass fast pyrolysis in N2 and CO2 atmospheres by thermogravimetric analysis [24]. Banana leaves were similarly pyrolyzed in the work by Sellin et al., with air as the fluidization agent [25]. Park et al. and Pighinelli et al. performed biomass pyrolysis works using a pilot-scale fluidized bed reactors, where the non-condensable gas was recycled [26,27].
This paper presents the results of the study that evaluated the fast oxidative pyrolysis of cassava pulp residue (CPR) in a fluidized bed reactor. Cassava (Manihot esculenta Crantz) is an important food crop in many countries, providing an essential part of the diet for majority of the population in Africa, Latin America and Asia. The crop also provides a livelihood for farmers, traders, and processors worldwide. Cassava is commonly consumed directly, or in the form of starch and flour. Residual pulp is separated from the starch in the screening process during the processing of cassava starch. The CPR is typically used as fodder.
The investigation operated on a hypothesis that recycling of uncondensed pyrolysis gases promotes the production of the biocrude product. The fluidized bed system was designed to recycle the uncondensed gaseous products, and use it as the fluidization gas to create a reactive environment for the biomass fast pyrolysis. The effect of the presence of this reactive atmosphere on the product yields and on the chemical composition of biocrude and charcoal products was studied for CPR
The CPR samples were obtained from a cassava starch processing plant located in Bukidnon Province in Southern Philippines. The biomass had a high moisture content (80 wt %), thus requiring sun drying for 3 to 5 days. After sun drying, the samples were milled to a particle size of around 2 mm and further oven-dried to a moisture content below 10 wt %. Proximate chemical analysis was conducted using thermogravimetry according to procedures described in ASTM 1762, while ultimate analysis was carried out according to ASTM D 5373 (for CHON) and ASTM D4239 (for S). The higher heating value (HHV) was measured using Parr 1341EB oxygen bomb calorimeter according to ASTM D 2015. These analyses are summarized in Table 1.
Table 1: Proximate and ultimate analyses of dried cassava pulp residue and other biomasses described in literature| Proximate analysis | Units | Cassava pulp residue | Sawdust [12] | Spent coffee grounds [28] | Sugarcane bagasse [29] |
|---|---|---|---|---|---|
| Moisture | wt % | 8.88 | 7.8 | 11.78 | 10 |
| Ash | wt % | 1.44 | 7.1 | 1.82 | 4.4 |
| Volatile matter | wt % | 81.67 | 82.9 | 68.94 | 76 |
| Fixed carbon | wt % | 8.13 | 10.0 | 17.46 | 9.6 |
| HHV | MJ kg-1 | 13.61 | 19.2 | - | 17.7 |
| LHV | MJ kg-1 | - | 17.7 | 18.11 | - |
| Ultimate analysis | |||||
| Carbon | wt % | 42.90 | 53.4 | 61.13 | 43.2 |
| Hydrogen | wt % | 5.72 | 8.0 | 8.99 | 6.2 |
| Nitrogen | wt % | 0.73 | 0.8 | 2.91 | 0.4 |
| Sulfur | wt % | 0.12 | 0.1 | 0.37 | 0.8 |
| Oxygen | wt % | 49.09 | 37.8 | 26.60 | 43.2 |
| C/H | 7.5 | 6.6 | 6.80 | 6.9 | |
| 0.9 | 1.3 | 2.29 | 1 |
As seen in Table 1, the dried CPR has physical and chemical characteristics similar to those of other agri-processing biomass studied in literature, such as sawdust, spent coffee grounds, and sugarcane bagasse. The dried CPR has a high volatile matter content (81.67 wt %) and low ash content (1.44 wt %), which can affect the product yields and characteristics of the biocrude and charcoal obtained in the pyrolysis [30,31].
The dried CPR was found to have low nitrogen (0.73 wt %) and low sulphur (0.12 wt %) contents, which are characteristic of wastes from the vegetal extracts industry. The C/H (7.5) and C/O (0.9) ratios are characteristically high [12,28,29,31]. The HHV of the dried CPR (13.61 MJ kg-1) is lower than those obtained from agri-processing waste biomasses, according to those presented in Table 1.
The thermal decomposition behaviour of the dried CPR was examined using thermogravimetric analysis (TGA) and differential thermal analysis (DTA). The samples were analyzed under N2 atmosphere, temperature range up to 800 °C, heating rate of 10 °C/min in a thermomechanical analyzer, Shimadzu TMA 60H. The TGA and DTA curves of the dried CPR are shown in Figure 1. As can be seen, weight loss underwent 3 main stages of decomposition. The first stage is the removal of water and lower molecular weight compounds, which occurred up to a temperature of 160 °C. In the second stage, maximum decomposition (82.8 wt%) was observed at temperatures from 200 °C to 470 °C, which was due to the decomposition of hemicellulose and cellulose (active pyrolysis stage). Hemicellulose decomposed at temperature 200 °C to 345 °C with the maximum weight loss attained at 330°C. Cellulose pyrolysis was focused at a higher temperature range (345-450 °C), with the maximum weight loss attained at 450 °C. The third stage is where lignin was decomposed until 800°C, which occurred at a slower rate. As observed from the TGA and DTA curves of the dried CPR, degradation was significant up to 500 °C, hence the biocrude production was set at about this temperature to attain the maximum degradation and volatilization of the biomass.
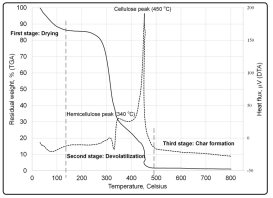
Figure 1: TG and DT profiles of dried cassava pulp residue
The fluidized bed reactor was used for the fast pyrolysis of the CPR, in which feedstock rate was adjusted to 1.59 kg h-1. Figure 2 shows the schematic layout of the experimental system, which is composed of a biomass hopper, a screw feeder, the fluidized bed reactor enclosed by an electric tube furnace, 2 sequential cyclones where charcoal particles are separated and stored in bins, a condenser where the gas/vapour mixture is condensed for biocrude recovery, and a recirculation system where the uncondensed pyrolysis gas is recirculated. Gas flow was controlled by a ball valve, and gas flow rate was measured using a Fox Model FT2A gas flow meter.
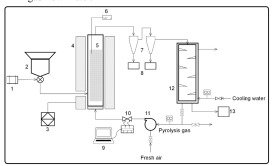
Figure 2: Schematic diagram of a fluidized bed system for the fast oxidative pyrolysis of cassava pulp residue. 1, motor; 2, feed hopper; 3, furnace temperature controller; 4, electric tube furnace; 5, fluidized bed reactor; 6, K type thermocouple; 7, cyclones; 8, charcoal collectors; 9, laptop; 10, laminar flow element; 11, blower; 12, condenser; 13, biocrude collector.
A gas distribution plate was placed at the bottom of the bed through which the fluidization gas flowed upward. The reactor was initially pre-heated by the electric tube furnace, until the silica sand (particle diameter between 0.60 mm and 0.85 mm) reached a temperature of around 500 °C. The CPR feedstock from the hopper was then introduced by a screw conveyor, to come in contact with the hot bed of silica sand that served as a heat transfer medium. CPR underwent pyrolysis in the reactor, producing volatile and non-condensable gaseous products, which were sent through 2 cyclones in series to remove the charcoal particles. The volatile and non-condensable gaseous mixture was cooled in a double-shell tube condenser with water at room temperature, forming the biocrude. The uncondensed pyrolysis gases were returned to the fluidized bed reactor by a blower with an inlet port connected to the condenser outlet. A gas outlet in the blower inlet line allows for the discharge of the returned gases to maintain a constant fluidization gas velocity
Three pyrolysis experiments were conducted at a temperature of 500 °C. To investigate the effect of pyrolysis gas recycling on products yields and characteristics, runs were carried out at different recycle rates, ranging from 0.6 to 2.3 Sm3 h -1. The air flow rate into the reactor ranged from 14 to 26 m3 h -1, corresponding to equivalence ratios (ERs) between 0.08 and 0.15, was maintained during the experiments. ER is defined as the ratio between the flow rate of oxygen in the air used in the experiment and the stoichiometric flow rate required for the complete combustion of the biomass.
The condensed products, specifically the biocrude, were collected in the condenser and subsequently weighed to obtain the mass of biocrude. The mass yields of the products were determined as the ratio between the mass of each product after fast pyrolysis and the mass of the biomass fed to the process. Charcoal collected at the cyclones and from inside of the reactor at the end of the pyrolysis run were weighed. Biocrude and charcoal yields were calculated by dividing the mass of the desired product by the mass of the CPR feedstock. The gas yield was determined by difference, i.e. gas yield = 100 - (biocrude yield + charcoal yield). The mass of the recycled pyrolysis gas was determined from the value obtained from the flow meter reading.
Analysis of the chemical compounds present in the biocrude was carried out by Fourier Transform Infrared Spectroscopy (FTIR) in a Perkin Elmer Spectrum 100 spectrophotometer, using the Attenuated Total Reflection (ATR) accessory. The following biocrude properties were analyzed: The HHV was measured using Parr 1341EB oxygen bomb calorimeter according to ASTM D240, TAN (in mg KOH per g sample) was determined according to ASTM D974, viscosity according to ASTM D445, and flash point using Cleveland open cup tester according to ASTM D92.
The charcoal produced from the pyrolysis experiments was characterized by proximate chemical analysis and HHV using the same procedures as those used for the dried CPR. Surface morphological changes of the charcoal sample were investigated using a scanning electron microscope (Hitachi SU 3500). The uncondensed pyrolysis gases were analyzed using an Agilent 7820A gas chromatograph equipped with a thermal conductivity detector (TCD) and flame ionization detector (FID). The concentrations of hydrogen (H2 ), carbon monoxide (CO), carbon dioxide (CO2 ), nitrogen (N2 ), methane (CH4 ), ethane (C2 H6 ) and propane (C3 H8 ) were determined.
As mentioned above, fast pyrolysis process was carried out to investigate the influence of the reactive (i.e. reducing) atmosphere, formed by the recycled uncondensed pyrolysis gases, on the product yields and characteristics. Figure 3 shows the compositions of the gases generated from the three experiments. It is observed that more than 50% of the gas produced from the fast pyrolysis was composed of N2. Concentrations of hydrocarbon gases were reduced, while CO2 and CO were increased when recycle rate was increased (and ER was decreased). At a recycle rate of 0.60 Sm3 h -1, around 1.5% of the fast pyrolysis gas was H2 . The H2 concentration was found to be considerably reduced when recycle rate was increased
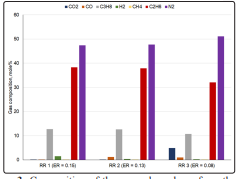
Figure 3: Composition of the uncondensed gas from the fast pyrolysis of cassava pulp residue RR1, recycle rate = 0.60 Sm3 h-1; RR2=0.95 Sm3 h-1; RR3 = 2.3 Sm3 h-1
The properties of fast pyrolysis biocrude from CPR at a recycle rate of 2.3 Sm3 h -1 (ER = 0.08) were determined and are presented in Table 2, in comparison with those obtained from other biomasses described in literature. The viscosity at 25 °C, which is considerable lower than the values from Park et al. and Pighinelli et al., is in agreement with the work of Abnisa et al. [26,27,32]. The presence of water in the biocrude affected its viscosity, which has been shown to decrease with an increase in water content [26,32]. The water content of the biocrude was not determined in this study. However, since the amount of feedstock and moisture in the feedstock was comparable for all three experiments, it is expected that the water content would be lowest in the biocrude product from the fast pyrolysis using the highest recycle rate. Water in the biocrude came from the moisture in the CPR feedstock and air, from the dehydration of cellulose and hemicellulose during the pyrolysis process, and from the combustion of recycled pyrolysis gas and charcoal. In the experiments, biocrude viscosity is expected to increase with increasing recycle rate. While high water content improves biocrude flow (reduces its viscosity), which is helpful for pumping and atomization, it causes a reduction in its heating value and flame temperature, and leads to ignition difficulties. The heating value of the CPR biocrude was 12.7 MJ per kg, consistent with the values obtained by Park et al. and Weerachanchai, Tangsathitkulchai and Tangsathitkulchai [26,33].
Table 2: Properties of biocrude from pyrolysis of cassava pulp residue and other biomasses described in literature| Units | Cassava pulpresidue biocrude | Empty fruit bunch biocrude [26] | Sawdust biocrude [26] | Sugar cane trash biocrude [27] | |
|---|---|---|---|---|---|
| HHV | MJ kg-1 | 12.7 | 13.2 | 15.9 | 29.86 - 32.75 |
| Kinematic viscosity | mm2 s-1 | 1.31 | 14.5 | 21.7 | 62.54 - 74.76 |
| TAN | mg KOH g-1 | 13.70 | 100.0 | 75.6 | 16 - 20 |
| Flash point | °C | 120 | 46 | 49 | - |
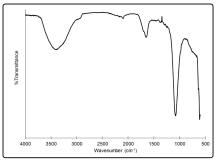
Figure 4 shows the biocrude spectrum from the FTIR analysis. The rounded absorption peak observed between 3,200 and 3,600 cm-1 indicates the presence of the OH groups, i.e. water, alcohols and phenols. The peak at 1,650-1,750 cm-1, which is attributable to C=O stretching, indicate the presence of carboxylic acids, ketones, and aldehydes. Alcohols and phenolic groups give rise to the strong C-O stretching mode around 1,000 cm-1. The C-H bending mode near 610 cm-1 was assigned to alkynes
The charcoal particles keep the original shape of the CPR particles, although they appear to be more porous and have a higher fixed carbon content. SEM micrographs of the raw CPR biomass and the charcoal obtained from fast pyrolysis of CPR at 2.3 Sm3 h -1 recycle rate (ER = 0.08) are shown in Figure 5. As can be seen from the images, the spherical structures on the surface of the raw CPR were transformed into pores and holes after pyrolysis. The change in the primary surface structure is likely due to the devolatilization of volatile matter during the second phase of the pyrolysis process.

Figure 5: SEM micrographs (magnification 1000x) of (a) raw cassava pulp residue (CPR) and (b) CPR charcoal obtained from fast pyrolysis
The proximate analysis and HHV of the charcoal from fast pyrolysis of CPR at 500 °C and a recycle rate of 2.3 Sm3 h -1 (ER = 0.08) and other biomasses described in literature are presented in Table 3. Fixed carbon content agrees with values obtained for sugar cane trash. Volatile matter is lower than that of the banana leaves charcoal, but higher than that of the corn stover and sugar cane trash charcoals. The low volatile matter and high fixed carbon are likely the result of the thermal degradation of the CPR during fast pyrolysis. Moisture and ash contents are considerably low compared to other biomass charcoals. Moisture content of the CPR charcoal decreased when compared to the CPR feedstock, which could be attributed to the dehydration phase of the pyrolysis process. As expected, the ash content of the CPR charcoal was higher than the CPR feedstock, because the inorganic matter originally present in the biomass remains in the charcoal after pyrolysis. The charcoal obtained has a heating value that is about 73 percent higher than that of the CPR feedstock. The low moisture and relatively high volatile matter contents likely contributed to the HHV of the CPR charcoal. These results suggest that the charcoal from the fast pyrolysis of CPR is a suitable replacement for solid fuels.
Table 3: Proximate analysis of charcoal from pyrolysis of cassava pulp residue and other biomasses described in literature| Proximate analysis | Units | Cassava pulpresidue charcoal | Banana leavescharcoal [25] | Sugar cane trash charcoal [27] | Corn stovercharcoal [37] |
|---|---|---|---|---|---|
| Moisture | wt % | 0.91a | 1.68 | 2.45-3.26 | 1.0c |
| Ash | wt % | 3.16a | 23.5b | 19.44-21.56b | 49.7c |
| Volatile matter | wt % | 28.50a | 53.2a | 13.65-15.07a | 14.9c |
| Fixed carbon | wt % | 67.23a | 23.2a | 60.11-64.45a | 34.4c |
| HHV | MJ kg-1 | 23.59a | 18.2c | 31.73-32.14a | 13.83c |
a dry ash free (daf) basis
b dry basis (db)
c as received basis
Pyrolysis experiments were conducted at 500 °C, and gas recycle rates ranging from 0.60 to 2.3 Sm3 h -1. The influence of gas recycling on the mass yields of the products of the fast pyrolysis of CPR are presented in Table 4. Results of the experiments suggest that product gas recycling affects the biocrude and charcoal yields.
Table 4: Effect of uncondensed pyrolysis gas recycling on the yields of the different cassava pulp residue fast pyrolysis products at 500 °C (g per 100 gram biomass)
| Uncondensed gas recycling rate (RR), Sm3 h-1 | |||
|---|---|---|---|
| Pyrolysis product | 0.60 | 0.95 | 2.3 |
| Biocrude | 14.32 | 14.65 | 18.08 |
| Charcoal | 27.17 | 25.03 | 20.68 |
| Pyrolysis gas | 58.51 | 60.32 | 61.24 |
Some authors studied the influence of an oxidative environment on the pyrolysis product yields, and reported contradicting results [31,38,39]. For example, Amutio et al.reported that biocrude yield increased, while charcoal yield decreased, the more O2 is added to the reaction environment during the flash pyrolysis of forest pinewood waste in a conical spouted bed reactor [38]. On the other hand, Mesa-Perez et al. reported decreases in both the liquid and solid products of the pyrolysis of sugar cane straw in a fluidized bed reactor [31]. Jiang et al. similarly reported a reduction in the biocrude and charcoal yields with an increase in the oxygen concentration in the pyrolysis of mallee wood biomass [39]. The differences in the results can be attributed to the differences in the feedstock source and the reactor type.
A similar conflicting trend is observed in the present work. Air flow to the reactor was maintained during the experiments to guarantee the fluidization of the bed materials. The lower the gas recycle rate used, the more amount of air (and hence, more O2 ) was added to reaction environment. As can be seen in Table 4, biocrude yield decreased while charcoal yield increased with decreasing recycle rate (or increasing ER). The observed reduction in the biocrude yield at reduced recycle rate may have been due to secondary cracking of the liquid product, resulting from the introduction of more O2 . Hence, maximum biocrude production can be attained if gas recycle rates are increased beyond the highest rate used in this study, as had been demonstrated in previous studies [26,27]. Charcoal yield decreased in reductive pyrolysis, which can be attributed to its reaction with CO2 as evidenced by the increase in CO yield under high CO2 atmosphere. This result is in agreement with those reported by Zhang et al. [23].
The biocrude yields obtained in this study, under similar conditions (temperature and reducing atmosphere), are almost equal to the values obtained by Suntivarakorn et al. [40], but are lower than those obtained by Park et al.and Pighinelli et al. [26,27]. The difference that distinguishes the results between the latter and the present work is relative amounts of recycle gas, and their composition, present in the reaction atmosphere. While the recycle gas ranged between 2% and 15 vol% of the reaction atmosphere in this work, Park et al. recycled 80% of the uncondensed pyrolysis gas while recycle gas was 50% to 70% of the reactive environment in the work of Pighinelli et al. [26,27].
Fast pyrolysis of CPR was accomplished in a fluidized bed reactor at 500 °C, and the yields of the pyrolysis products were determined and their properties analysed. The partial recycling of the uncondensed pyrolysis gases, which also served as the fluidizing gas, contributed to form a reducing atmosphere in the reactor. The uncondensed gas was composed of more than 50% N2 when recycle rates ranged between 0.60 to 2.3 Sm3 h -1 (ER between 0.08 and 0.15) It was observed in this study that biocrude yield increased from 14.32% to 18.08% in the recycle rate range studied. The biocrude product was mainly composed of alcohols, phenols, aldehydes, ketones, alkanes, and alkynes. Biocrude fuel properties, i.e. viscosity and TAN, will likely be affected by its water content. Therefore, high recycle rates should be used, which will constrain the combustion of H2 . A further study of the recycle gas-air ratio vis-a-vis feed rate of biomass used in the reductive fast pyrolysis process, as well as the improvement of biocrude condensation process, can improve biocrude yield and properties. Additional biocrude upgrading process is also needed to make it suitable for use in industrial burners. Charcoal yield decreased from 47.47% to 40.98% under the reducing atmosphere, likely because of its removal from the reaction environment by its reaction with CO2 . The low moisture content and high volatile matter and fixed carbon contents, coupled with the high HHV, of the charcoal samples makes it suitable as a solid fuel. Energy and economic analyses will be necessary to determine the viability of converting CPR through the reductive pyrolysis route [40].
The authors thank Joeri Gomez and Danilo Ortizano (Phil-Agro Industrial Corporation) for providing the cassava pulp residue used in this study. This work was partly supported by the Kinaadman University Research Office (KURO) of Xavier University.
