Author(s): Baby Naz
The rapidly growing demand for electric vehicles and the large body of knowledge generated by the semiconductor industry are driving the rapid advancement of battery technology. By 2030, sales of new automobiles will be virtually entirely made up of electric vehicles (EVs). Next-generation batteries aim to target EV adoption barriers such as cost, carbon footprint, and range per charge. To fulfil sustainability goals, combined with reuse and recycling, enhanced battery lifecycle management and data collection/analysis are necessary. Additionally, firms in the semiconductor ecosystem are well-positioned to contribute to the development of the next generation of batteries because of the strong relationships between semiconductors and automotive batteries. The new battery pack being created by NASA researchers is lighter, safer, and performs better than batteries commonly used in vehicles and large electronics today. After
several years of successful work by a NASA activity called the Solid-state Architecture Batteries for Enhanced Rechargeability and Safety, the topic has now piqued the interest of the government, business, and academia (SABERS). Battery performance is critical in the development of more environmentally friendly electric aircraft.
Lithium-ion battery technology has truly changed our world by enabling reliable, long-lasting energy storage for off-grid applications. Even with these advancements, there is still room to improve the lithium-ion battery. The development of the solidstate battery is one advancement that could once again change the way we store energy [1]. Many of the problems associated with liquid Li-ion batteries, such as flammability, limited voltage, unstable solid-electrolyte interphase formation, and poor cycling performance and strength, may be addressed by solid-state batteries [2-3]. The replacement of volatile and flammable liquid electrolytes (LEs)used in conventional Li-ion batteries (LIBs) with nonfarmable solid electrolytes (SEs) is almost universally expected to improve safety A lithium-ion battery is composed of a cathode, anode, separator and electrolyte [4]. A lithium-ion battery applied at smartphones, power tools and EVs uses liquid electrolyte solution [5]. A solid-state battery is a type of battery that employs solid electrodes and a solid electrolyte rather than the liquid or polymer gel electrolytes found in lithium-ion or lithium polymer batteries [3]. Solid-state batteries utilize a solid material to allow energy to flow from the cathode to the anode [6]. Materials proposed for use as solid electrolytes in solid-state batteries include ceramics (e.g., oxides, sulfides, phosphates), and solid polymers [2,3] Click or tap here to enter text..
Solid-state batteries are inherently safer and more energy-dense than today’s lithium-ion batteries, which rely on flammable liquid electrolytes for fast transfer of chemical energy stored in molecular bonds to electricity [7]. The battery incorporates a solid-state electrolyte as well as an allsilicon anode, resulting in a silicon all-solid-state battery. Initial tests indicate that the new battery is safe, long-lasting, and energy dense. It has great potential for a wide range of applications, including grid storage and electric vehicles [2].
Solid electrolytes, a carbon-free anode, and a cathode composite layer are used in all-solid-state batteries (SSBs). Ions migrate into the ionically conductive solid matrix rather than the ionic salt dissolved in the solution during charge or discharge. Solid-state batteries use redox reactions to store and distribute energy. The cathode is reduced, and the anode is oxidised, allowing the battery to store and release energy as needed. In contrast to the polymer gel or liquid electrolyte used in traditional lithium-ion batteries for electric vehicles, solid-state batteries use a solid electrolyte made of glass, ceramics, solid polymers, or sulfites (EVs) [8].
A solid-state electrolyte (SSE) is a solid ionic conductor and electron insulator that is a key component of a solid-state battery. It is useful for applications in electrical energy storage (EES), particularly in the replacement of liquid electrolytes found in lithium-ion batteries [1,5]. The main advantages are absolute safety, no issues of leakages of toxic organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability [2,3]. Because of the property of lithium dendrite suppression in the presence of a solid-state electrolyte membrane, this allows for the use of a lithium metal anode in a practical device without the inherent limitations of a liquid electrolyte [3]. SEs allow Li-metal anodes to replace graphite anodes, increasing energy density over a traditional LIB. 2 Because it tends to deposit in mossy or dendritic microstructures that consume electrolyte and Li inventory, Li metal is difficult to cycle efficiently and safely with LE. 4,5 Dendrites grow easily through the separator, causing internal short circuits. 6,7 Polymer SEs with a high enough shear modulus are thought to inhibit dendritic growth and prevent short circuits. 8,9 This theory, however, does not apply to ceramic SEs, where grain boundaries provide growth pathways and fracture is a critical failure mode. Many studies have shown that Li dendrites can grow through SEs and short circuit cells [4].
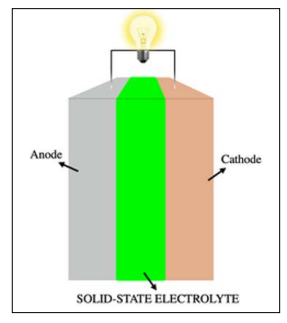
Figure 1: All-Solid-State Battery with the solid-state electrolyte [3].
Engineers at UC San Diego have collaborated with LG Energy Solution to create a new rechargeable solid-state battery. Scientists combined a solid-state sulfide electrolyte and a silicon anode in a single device, omitting lithium and carbon entirely. During testing, the battery demonstrated its safety, durability, and high energy intensity. At room temperature, the prototype withstood 500 charge and discharge cycles while retaining 80% capacity. The technology opens up exciting possibilities for electric transportation, energy storage, and other fields [9].
Lithium (Li) is present in both solid-state and lithium-ion batteries; in both, the Li+ ions move from one part of the battery to another, allowing negatively charged electrons to move through a circuit. The main distinction is what the ions pass through. A liquid electrolyte is used in a traditional lithium-ion battery, whereas a solid material is used in a solid-state battery. They are technically more difficult to manufacture, but they may be more efficient than standard lithium batteries [10].
Every battery has two electrodes: an anode (the negative side) and a cathode (the positive side). These two electrodes are made of a material that conducts electricity.
An electrolyte containing electrically charged particles is present between (and within) these two electrodes (ions). Lithium ions can travel through the electrolyte and combine with the anode or cathode (depending on charging or discharging). This chemical reaction allows electrical charge to flow between the cathode and anode (via a circuit), allowing a battery to produce an electric current to power your device [1].
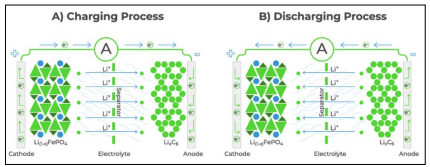
Figure 2: The charge and discharge process of a LiFePo4 battery works by moving Lithium Ions across the electrolyte while electrons pass through the circuit [1].
The electrodes and electrolytes in solid-state batteries are both solid-state,” according to IDTechEx. “Solid-state electrolytes normally behave as the separator as well, allowing downscaling due to the elimination of certain components, such as the separator and casing. As a result, they may be thinner, more flexible, and contain more energy per unit weight than conventional lithiumion batteries. The removal of liquid electrolytes can also lead to safer, longer-lasting batteries that are more resistant to temperature changes and physical damage during use [11,12].
There are multiple benefits to solid-state battery technology. In short, the solid electrolyte used in a solid-state battery provides higher energy density, longer lifespan, and increased safety in a smaller size [1]. A solid-state battery with solid electrolyte shows improved stability with a solid structure, and increased safety since it maintains the form even if the electrolyte is damaged [5].
Market research firms predict that EVs will eventually replace ICEVs (internal combustion engine vehicles) and become the norm in the auto industry. To become the undisputed industry leader, EVs must have the same level of mileage as current ICEVs, which requires increasing the battery capacity of an EV battery. There are two methods for increasing capacity. The first step is to increase the number of batteries. However, in this case, the battery price rises, and batteries take up a lot of space in the vehicle. A solid-state battery has a higher energy density than a liquid electrolyte solution-based Li-ion battery. Because there is no danger of explosion or fire, there is no need for safety components [5].
A solid-state battery can increase energy density per unit area since only a small number of batteries are needed. For that reason, a solid-state battery is perfect to make an EV battery system of module and pack, which needs high capacity [5]. Solid-state batteries have found use in pacemakers, RFID and wearable devices. They are potentially safer, with higher energy densities, but at a much higher cost. Challenges to widespread adoption include energy and power density, durability, material costs, sensitivity and stability [2]. Let’s take a closer look at how this translates to how a user will benefit from solid-state battery technology [1].
Energy density is a measurement of how much energy a battery contains in relation to its weight. Solid-state batteries are said to have 2.5 times the energy density of current lithium-ion technology. Because of the tremendous increase in the energy density of solidstate batteries, they will be much smaller and lighter. Higher energy density means batteries can be much lighter while still storing the same amount of energy. Mobile users are concerned about their weight. This could be a game changer in terms of power applications. Cars, trucks, RVs, boats, and aeroplanes could all benefit from weight reduction. Electric vehicles can benefit greatly from this technology as well, as they can get much more range with less weight and storage space [1].
Faster Charging Time: Solid-state batteries can work at very high rates of power. Research suggests that they may be able to recharge 4-6 times faster than current technologies safely [1].
Safer Battery Experience: The liquid electrolytes found in lithiumion batteries are extremely flammable and volatile. These electrolytes should also not be exposed to air. Solid-state batteries have no liquid components and will not contain this volatile component. While lithium-ion batteries are susceptible to events such as thermal runaway, which can result in explosion and fire, the solid electrolytes used in solid-state batteries are nonflammable, posing a lower risk of igniting a fire. While the battery may become hot, it contains nothing flammable that will catch fire. Furthermore, these batteries will necessitate fewer safety systems than lithium-ion batteries. Eliminating extra electronics can result in smaller, lighter-weight battery packages, which increases the energy density of solid-state batteries even more [1]. Solid-state battery technology is believed to deliver higher energy densities (2.5x). They may avoid the use of dangerous or toxic materials found in commercial batteries, such as organic electrolytes. [2]. Solid-state batteries are thought to have a lower risk of catching fire because most liquid electrolytes are flammable and solid electrolytes are nonflammable. Fewer safety systems are required, increasing energy density at the module or cell pack level even further. According to recent studies, heat generation inside conventional batteries with liquid electrolytes under thermal runaway is only 20-30%. Faster charging is expected with solid-state battery technology. Higher voltage and longer cycle life are also viable options [2,3]. These batteries may not even require an external BMS in certain situations, greatly simplifying its construction [1].
Much Easier to Manufacture: Working with a volatile liquid that cannot be exposed to air is a huge challenge that could be completely eliminated with solid-state battery technology. A solid electrolyte could enable much faster production that uses less material and energy [1]. Solid-state electrolytes are also advantageous for enabling next-generation batteries that use metals such as lithium and aluminium as anodes to achieve far greater energy storage than is currently possible with state-of-the-art battery technology. The solid-state electrolyte prevents the metal from forming dendrites, which can short-circuit a battery and cause overheating and failure[7].
All-solid-state batteries promise higher energy storage density, improved reliability and wear resistance, faster charging, and, most importantly, increased operational safety. Liquid electrolytes become volatile and flammable at high temperatures. Solid electrolytes, on the other hand, have high thermal stability, which reduces the risk of fire or explosion [8].
Because of their small size, solid-state batteries have a higher energy density per unit area. A solid-state battery’s energy density can be up to ten times that of a lithium-ion battery of the same size. Modern lithium-ion batteries for electric vehicles typically last between 2,000 and 3,000 cycles before deteriorating noticeably, whereas high-density solid-state batteries can last up to 10,000 cycles [8]. The new battery is secure, long-lasting, and high in energy density. Because solid electrolytes are non-flammable and more stable, they hold promise for a wide range of applications ranging from grid storage to electric vehicles.
Major users of solid-state batteries in the market are Solid Energy System, Cymbet, Robert Bosch GmbH, Toyota Motor Corporation, Bright Volt, Samsung SDI Co. Ltd, Solid Power, Excellatron Solid State, QuantumScape, Stmicro electronics N [3].
Toyota: Toyota, a Japanese manufacturer, has been monitoring the solid-state battery industry for years and even holds the most solid-state battery patents.
Samsung: Two years ago, Samsung introduced a high-performance and durable all-solid-state battery. The prototype battery can drive an electric vehicle up to 800 km on a single charge and has a lifespan of more than 1,000 charge cycles.
Nissan-Renault-Mitsubishi: Nissan, Renault, and Mitsubishi have announced a combined investment of €23 billion in electric vehicles [8].
QuantumScape: QuantumScape is widely regarded as a pioneer in the field of solid-state batteries. Volkswagen, Bill Gates, and SAIC Motors all support this San Jose, California-based corporation. QuantScape has already developed a solid-state battery that can charge from 0% to 80% in under 15 minutes, whereas a Lithiumion battery takes 60 minutes to charge from 10% to 80%. These batteries have an energy density that is 80% greater than Lithiumion batteries. [8].
Solid-state batteries have the potential to be safer, and they have the potential for higher energy density,” says Dr Alex Bates of Sandia National Laboratories in the United States. “According to some scientists, any amount of liquid electrolyte is dangerous. So, rather than simply accepting the ‘party line,’ we ran the numbers to see what the effects of liquid electrolyte might be.
The researchers put three types of batteries to the test: a solid-state battery, a lithium-ion battery, and a hybrid with varying amounts of liquid electrolyte. For each type of battery, they investigated three potential failure scenarios: external heating (read: fire), an internal short circuit, and mechanical failure (read: crushed or punctured battery).
In the fire scenario, the battery with a small amount of liquid produced about a fifth of the heat produced by a lithium-ion battery, while the solid-state battery produced no heat. In the internal short circuit scenario, all three batteries performed similarly
In a lab, two men in masks load a hand-sized battery into a spherical chamber. Alex Bates (front) and John Hewson are preparing a battery testing chamber. However, in the event of a mechanical failure, the pure solid-state battery may generate even more heat than its traditional counterpart [4].
In conclusion, solid-state batteries will be lighter, smaller, more powerful, charge faster, last longer, and be safer than conventional lithium-ion battery technology. Higher energy densities are expected from solid-state battery technology (2.5multiple). They may avoid using potentially hazardous or toxic materials found in commercial batteries, such as organic electrolytes.
Solid-state batteries have much higher thermal stability than lithium-ion batteries and can store 50% more energy. The solidstate battery is one of the most promising future battery solutions, with their high thermal stability making them significantly safer and longer-lasting than traditional electric vehicle batteries.
Solid-state batteries have long been regarded as the next step in electric vehicle development. They are less flammable, lighter, and store more energy than liquids. Until recently, there were two major impediments: the cost and durability of such batteries.
Solid-state batteries are said to be safe because the solid electrolyte is firm and unlikely to break. However, if it does break, the temperature rise could be comparable to that seen when lithium-ion batteries fail. They begin to degrade after several charge-discharge cycles due to the accumulation of lithium dendrites, which are tiny, twig-like lithium particles that grow and can penetrate the battery, causing short circuits and other problems. Once these issues have been resolved, a new battery will be installed.
