Author(s): Christine Blane
Background: There is currently no consensus as to the optimal imaging modality for Significant Polyps and Early Colorectal Cancer (SPECC). This study describes the use of transrectal ultrasound (TRUSS) as part of a quadruple assessment process to evaluate the depth of invasion of SPECC lesions and guide local excision.
Method: Pre-operative imaging and histology were reviewed for all patients undergoing transanal endoscopy microsurgery (TEMS) at Cheltenham General Hospital between 2013 and 2019. Treatment options included mucosectomy, partial thickness excision and full thickness excision. The dataset was studied specifically to evaluate the risk that malignancy will be present at the deep margin. ‘Failure’ of assessment was thus defined as the failure to detect and achieve complete local excision of malignancy.
Results: 400 patients were included: 319 primary lesions and 81 secondary lesions. Amongst the primary lesions 93.7% (n=299) had successful R0 excisions. Of the primary lesions with incomplete excisions 50% (N=10) had undergone mucosectomy, 40% (n=8) full thickness excision and 10% (n=2) partial thickness excision.
The potential for malignant transformation of colorectal polyps is well understood; however evaluating lesions remains challenging [1]. Size and complexity of a lesion increases its likelihood of malignancy, but as the focus of cancer is often at the base and often not detected on biopsy, differentiation of cancerous polyps remains challenging. Although approximately 10-15% of colorectal polyps >20mm are malignant, up to 90% will be benign and avoidance of unnecessary radical treatment/surgery is critical [2].
The term Significant Polyps and Early Colorectal Cancer (SPECC) has been introduced to describe the lesions. The detection rate of SPECC lesions is increasing as more people undergo bowel cancer screening and the overall incidence of colorectal cancer rises.
All of the therapeutic options for SPECC lesions include significant risks such as bleeding, perforation and pelvic sepsis. These risks are higher with deeper resections [3,4]. The widely accepted principles of ideal treatment are that patients should receive the least invasive treatment that will successfully achieve an R0 excision and that SPECC lesions should be resected “en bloc” with treatment to achieve a complete excision with clear margins (R0). This may be accomplished via a range of platforms offering mucosectomy and full thickness excision.
Therefore, the MDT must consider the depth of resection an individual lesion requires. Benign lesions require an excision in the submucosal plane only. For early malignant lesions, for those confined to the upper submucosa a non-full thickness excision may deliver an R0 excision, whereas those extending to the deep submucosa or beyond require full thickness excision to achieve R0. Once the deemed appropriate plane of excision has been selected, this can be delivered via a number of different platforms based on clinician experience, availability and preference. These include transanal operating platforms such as Transanal Endoscopic MicroSurgical (TEMS) for any plane and for non-full thickness excisions, endoscopic therapy such as Endoscopic Submucosal Dissection (ESD).
One essential aspect is the correct determination of the appropriate plane of excision. This is fundamentally different to the concept of ‘stage accuracy’ and is a key direction is which the assessment of these early SPECC lesions is moving. At present there is no widespread consensus as to the optimal pre-operative assessment modality for SPECC lesions although endoscopic assessment, MRI and transrectal ultrasound all have a role.
In this paper we will present our experience using transrectal ultrasound as part of a quadruple assessment to correctly select the plane of excision in SPECC lesions; to minimise the risk of incomplete excision (deep plane R1) of carcinoma (expected or unexpected) and obtain an R0 specimen.
Cheltenham General Hospital is a secondary and tertiary referral centre for SPECC lesions. In our practice, there is not only a large workload of primary SPECC lesions, but there are also a high number of lesions having undergone previous intervention with either residual disease or where the lesions is recurrent (months or years after previous intervention). These are particularly challenging but an essential part of the workload of the advanced SPECC MDT
Patients referred for consideration of treatment undergo a quadruple assessment, which includes clinical history, digital rectal examination, flexible sigmoidoscopy and transrectal ultrasound scanning (TRUS). These are performed at one appointment, in the Endoscopy Department under phosphate enema preparation without routine sedation. The TRUSS is performed using the 3D ultra BK 3000 system with a 20R3 transducer at 13MHz probe setting. Patients referred from elsewhere may have already had an MRI, but local policy involves rectal MRI scanning for cases where established disease or intra-mesorectal disease is suspected, often after quadruple assessment. During the period of study, the prime modality for treatment of SPECC lesions was TEMS excision. More recently formal ESD has been available but was not part of treatment during the study period.
All patients are added to a prospective database at the time of initial assessment. The following recorded data were derived from the patient data recorded:
The predicted stage of the lesion. uT0 is used for lesions considered non-invasive. The terms uT1, uT2 and uT3 have been used throughout due to convention for recording stage of lesions considered invasive in this fashion. An estimate of depth of T1 invasion (sm1/2/3) was not routinely recorded throughout the series but was in the later cases.
In a small number of cases uncertainty of assessment as to the depth of invasion of a particular lesion was recorded (e.g., ‘deep T1, possibly T2). but a final assessment result was documented pre-operatively in each case. Where this occurred, cases were analysed according to the least invasive predicted depth of invasion. Where no reliable assessment of depth of invasion could be achieved either due to inability to acquire adequate TRUS images or inability to determine the appearances from the images. The intended/recommended depth of excision to achieve an R0 excision based on the assessment was also recorded.
All the TRUS procedures were carried out by a single consultant surgeon with 12 years’ experience in this technique and in TEMS procedures at the start of the time period chosen. This time frame has been chosen so as to be confident that this consultant is well beyond the learning curve of the technique. The intended depth of excision was included in the TRUS report.
All treatments were reviewed and confirmed at an MDT meeting (early in the series the specialist Early Rectal Cancer MDT at which complex benign lesions were also reviewed) and latterly the specialist SPECC MDT. Where present, pre-operative MRI results were compared to TRUS imaging. Where the MRI and TRUS findings were discordant, treatment decisions were made on the basis of quadruple assessment MRI scans were only taken into consideration, if present, where they demonstrated evidence of rT3 invasion or intramesorectal disease.
Rectal lesions of all sizes were considered for excision including putatively benign lesions, suspected early cancers, lesions in patients who had undergone previous local interventions, recurrent lesions and very large lesions. Treatment throughout the study period was usually by a mucosectomy or full thickness excision using the Transanal Endoscopic MicroSurgery (TEMS) platform. Partial thickness excision was defined as inclusion of part of the muscularis propria or scar tissue, if present in recurrent lesion, deep to the submucosa but without formal full thickness disc excision and without suture closure.
Final pathology results were reviewed at the specialist MDT(s) following treatment. The final histolopathology and depth of invasion (non-invasive, pT1, pT2, pT3 and where appropriate sm1/2/3) were recorded for all lesions. The margin of excision was recorded both for circumferential and deep margins. For this study, the prime margin of interest was the deep margin, specifically for invasive lesions. This was used to determine completeness of excision (R0)
Histology reports were reviewed, and invasive malignancy seen within 1mm of the margin was classed as an R1 resection even though some authors have suggested that only actual microscopic involvement of the diathermised margin should be considered as R1 (WEST et al 2018, ACPGBI guidelines).
We performed a retrospective analysis of the notes, imaging and histology results of all patients undergoing TEMS excision at Cheltenham General Hospital (CGH) between November 2013 and December 2019. Patients were identified from the Cheltenham SPECC database. It was beyond the scope of this study to consider patients pursuing other treatment modalities. Any patients whose data was incomplete were excluded from the study.
All the lesions were categorised based on their predicted depth of invasion at TRUS- uT0 (benign), uT1, uT2, uT3. Lesions whose stage could not be predicted by TRUS were classified as uTX, this included those where scanning was incomplete or satisfactory images could not be obtained. Any queries regarding proposed treatment in the preoperative documentation were clarified with the operating surgeon. Patients with recurrent lesions or who had undergone previous local interventions were classed as secondary lesions.
Any discordance between the planned depth of resection and the depth of resection described in the operation note was recorded. Results have been analysed on the basis of the intended depth of resection.
The dataset was studied specifically to address the following questions: A -For primary lesions, where assessment leads to a recommendation for mucosectomy (non-full thickness) excision, what is the risk that malignancy will be present at the deep margin (‘R1 Ca’). B - For primary lesions where assessment leads to recommendation for full thickness excision what is the risk of ‘R1 Ca’ C - For Secondary lesions how accurate is assessment at direction plane of excision (non-full thickness or full thickness) in avoiding ‘R1 Ca’
‘Failure’ of assessment was thus defined as the failure to detect and achieve complete local excision of malignancy
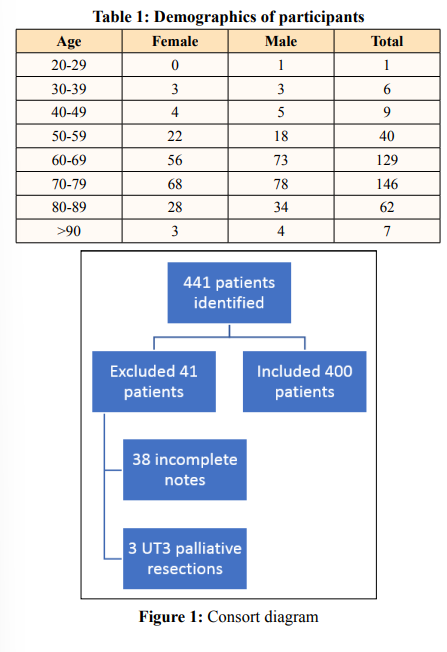
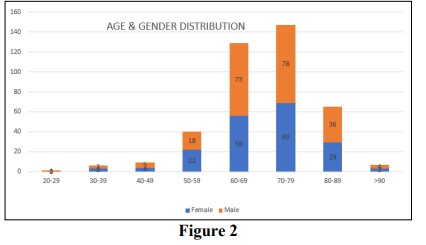
A total of 441 TEMS excisions were performed during this period November 2013 to December 2019. Data was incomplete for 38 patients (8.6%) who were then excluded from the study. The majority were because it could not be determined from the clinical and MDT notes what the single final decision was for plane of excision. A further 3 patients (0.7%) were staged at uT3 and received palliative excision for symptomatic disease and have also been excluded. The remaining 400 patients were all included (see Figure 1). There were 216 male patients and 184 female patients with a median age of 70 years old in a distribution set out in Table 1 and Figure 2. The youngest patient operated on was 29 and the oldest was 101. As can be seen, the majority (68%) of patients were aged between 60 and 79 years old, but a sizeable number (18%) were 80 or older. The data were analysed in two groups, those with primary versus those with secondary lesions.
Overall actual depth of excision matched intended depth of resection in 96.3% (n=385). Where discordance was noted, 12 received a deeper resection than intended and 3 received a shallower resection. From the operative notes the majority of the former were due to a suspicion of more advanced malignancy than assessed and the former due to low or anterior lesions where full thickness was deemed more hazardous.
There were 319 primary lesions in our analysis and overall, 93.7% (n=299) had R0 excisions. Lesions where staging could not be determined (uTx) are included in these analyses since the this is an essential part of the understanding of an assessment modality in the functioning of a SPECC MDT.
Primary lesions - mucosectomy intention
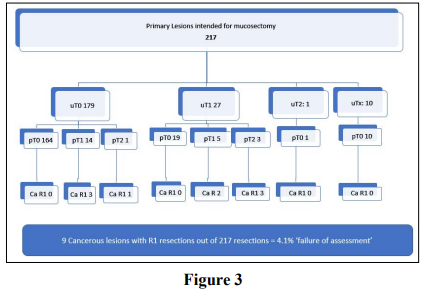
These are described on Figure 3.
Over two thirds of patients with primary lesions (n=217 68%) were
intended for mucosectomy. Approximately 10% (n=23) this group
had malignant lesions on pathology of which 14 (61%) had R0
excisions. The overwhelming majority (95.9%) n=208 of patients
undergoing mucosectomy for a primary lesion had R0 excisions.
The ‘failure’ of assessment to determine this plane of excision was thus 4.1%. Nine mucosectomies were incomplete excision of lesions with a malignancy. Of these, 4 cases were fully excised but with a clearance of less than 1mm so are still considered to be an R1. 1 case was fully excised but in a piecemeal fashion and therefore considered an R1 resection. In only 4 cases was there direct involvement of the diathermised margin, ‘failure’ by this definition thus occurred in only 1.8% of cases.
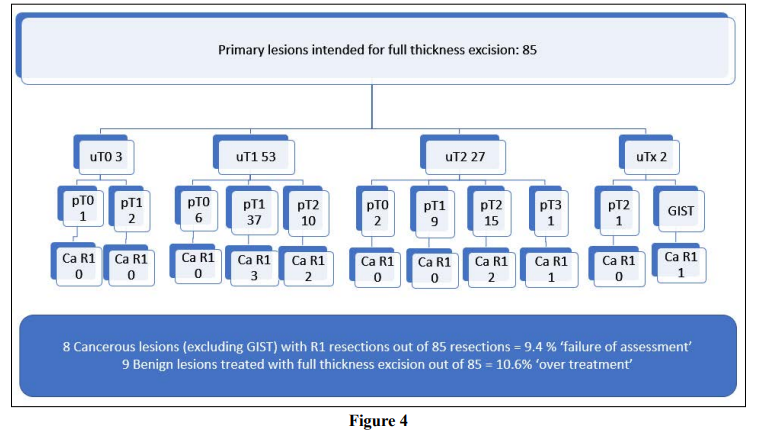
The results are shown in Figure 4. Full thickness excision was planned for 85 patients with primary lesions (26.6%). Pathology confirmed adenocarcinoma in 88% (n=75) of this group and one GIST. Failure, as defined by R1 resection of an adenocarcinoma occurred in 9.4% (n=8). Of these cases, 7 were fully excised but with a margin of less than 1mm. Only one case was involved of the deep margin. ‘Failure’ by this definition thus occurred in only 1.2% of cases with this intention.
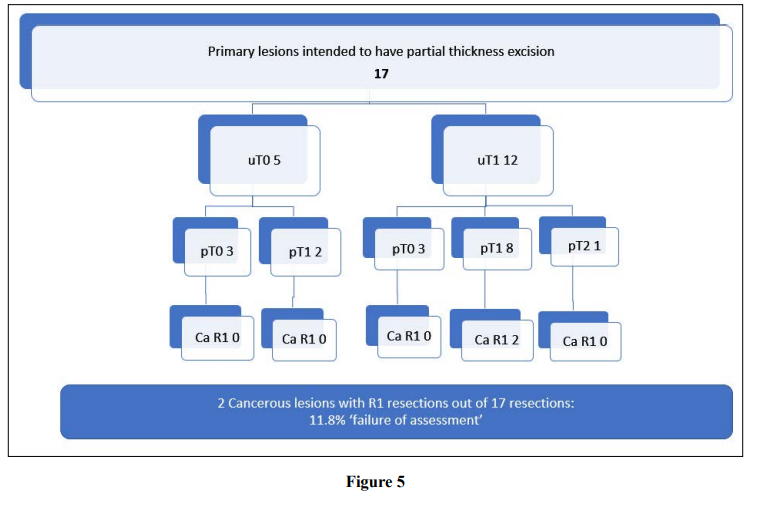
Partial thickness excision was planned for the 5.3% of primary lesions (n=17) as shown in Figure 5. This was usually due to a very low height or anterior position. Only 2 resulted in an R1 excision for cancer (‘failure’ rate of 11.8%), of which one was fully excised with a margin of 0.6mm and the other case was involved of the deep margin. The number of these cases, however, was small.
There were 81 secondary lesions in our analysis and overall, 92.6% (n=75) had R0 resections. The secondary lesions were more difficult to assess using TRUS. In fact, 66.7% (n=54) were classed as uTx compared with only 12 out of 319 patients (3.8%) with primary lesions. This is entirely expected given that the majority of these cases had either features of recent intervention or endomural/ perimural scar present from previous interventions making the determination of intact planes considerably more difficult. Secondary lesions were more likely to receive full thickness excision: 42.0% (n=34) compared to 26.6% (n=85) of primary lesions which may reflect this uncertainty. [In addition, a number were performed due to an R1 (incomplete) excision of an unexpected cancer by other procedures and thus confirmation of ‘no residual disease’ was often part of the treatment strategy.]
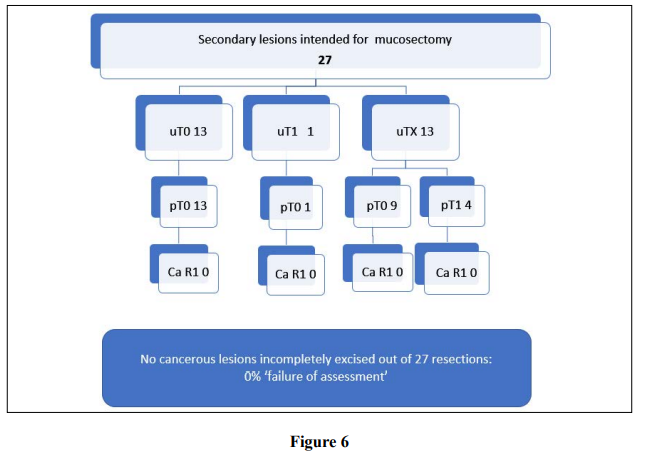
A third of second lesions 33% (n=27) were excised via mucosectomy; these are described on Figure 6. 4 patients had malignant lesions of which all (100%) were R0 resections with no instances of ‘failure’ to deliver R0 for malignancy although the numbers in the group are small.
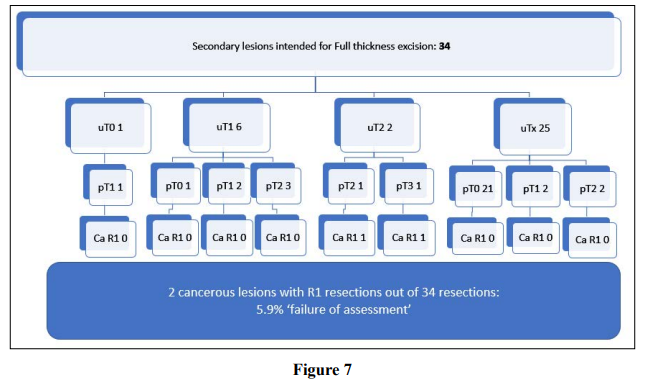
Full thickness excision was planned for 42% (n=34) secondary lesions; these are described on Figure 7. Overall, 94.1% (n=32) were R0 resections. Pathology confirmed malignancy in 35.3% (n=12). Only 2 cases (5.9%) had R1 resections for cancerous lesions, of which both were fully excised but with margins of less than 1mm.
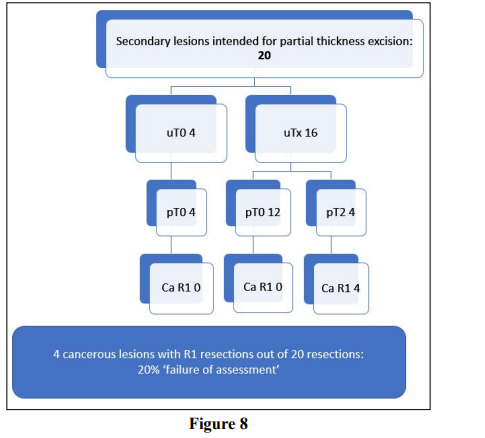
Partial thickness excision was planned for 25% (n=20) secondary lesions; these are described on Figure 8. Overall, 60% (n=16) were R0 excisions. Malignancy was confirmed in 20% (n=4) and all of these were unexpected pT2 disease with involved deep margins; 20% (n=4) ‘failure’ although the numbers are small.
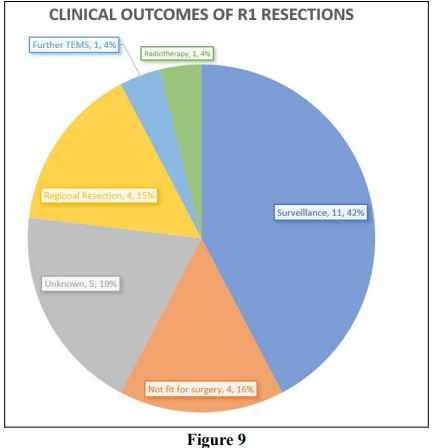
There were 26 patients who had incomplete resections for malignant lesions (25 adenocarcinomas and one GIST). The clinical outcomes for these are described in Figure 9. Only six patients are known to have received further treatment for their early rectal cancers, 4 of whom had either an anterior or abdominal perineal resection, 1 patient had radiotherapy and 1 patient had a further TEMS which was clear. 15 patients are known to have had no further treatment, 11 of whom were simply surveilled and 4 of whom were unfit for any kind of further treatment. Unfortunately, 5 of the patients were referred into CGH from other centres and information regarding their ongoing treatment could not be obtained.
At present there is no consensus on the optimal imaging modality for assessment of SPECC lesions. The accuracy of MRI and transrectal ultrasound have traditionally been assessed with reference to their ability to predict the T stage of an individual lesion; Within this paradigm both modalities have been observed to vary widely.
MRI has struggled to deliver accurate information on the T stage of an individual lesion. A recent Dutch population-based study (5539 participants) revealed that over 50% patients with early rectal cancer who underwent clinical assessment with MRI alone were overstaged, limiting the use of local excision [5]. Data from USA suggests that routine pre-operative MRI correctly stages approximately 60% T1 tumours [6]. Research is ongoing that seeks to determine the efficacy of a novel staging proforma for MRI in identifying possible planes of excision for some such lesions although it excludes many of the larger more complex lesions which pose the greatest challenge diagnostically and technically [7].
A large meta-analysis (42 studies, 5039 patients) evaluating TRUS described a sensitivity of >80% and a specificity of >90% for T staging however individual studies have recorded sensitivity as low as 50% [8]. Recent comparative studies suggest TRUS outperforms MRI in staging early rectal cancer and its role in differentiating between T1 and T2 tumours has been advocated by the European Society of Gastrointestinal and Abdominal Radiology (2016) [9].
Ultrasound is known to be a user dependent technology and some have called for its use to be restricted to high-volume, specialist centres [4]. It is important to note that in this case series TRUS was performed by an experienced consultant surgeon with a specialist interest in this field. It is also important to note that imaging is one part of the quadruple assessment process and the findings must be interpreted within the context of history, examination and endoscopic evaluation.
Rather than focusing on the ability of imaging to accurately predict the T stage of an individual lesion, it is our assertion that the paradigm for assessment needs to change. The value of TRUS or any other modality as an assessment tool should be judged on its ability to enable the clinician to deliver the correct treatment, rather than its concordance with the pathological T stage of the lesion. In most cases this is correct treatment is predominantly to obtain an R0 excision of cancer if present (expected or unexpected) whilst offering the least invasive excision (including shallowest depth of excision).
In this study this was achieved in over 92% both primary and secondary lesions. Included in this number were 66 lesions which could not be given a precise T stage at TRUS but for whom a determination on recommended depth of resection was given. It is essential to include these patients in analysis since this indicates the ‘real world’ reality of the complexities of staging and the overall experience of patients. This compares favourably with data from other UK centres who achieved negative resection margins in 91.5% cases and national data from USA which describes 84.1% R0 excision for patients undergoing local excision between 2008-2016 [6, 10].
Overall, 26 (6.5%) patients had an R1 resection. Of these 57.7% (n=15) were classed as R1 because the resection margin was <1mm from the lesion and in one case piecemeal excision. Some have suggested that this level of clearance may not actually be appropriate for early tumours but NHS Bowel Cancer Screening pathology reporting guidelines still require 1mm clearance [11]. Patients with prior intervention are much more difficult to assess and appear technically more challenging in terms of achieving clear margins. Patients with an R1 resection were offered salvage surgery, with or without chemotherapy, or ongoing surveillance.
The highest rates of treatment failure within this dataset were observed in cases where a partial thickness excision was performed, this was the case in both primary and secondary lesions although the numbers were small in both these groups. Mucosectomy is an established treatment option for putatively benign lesions although some centres advocate full thickness excision of all lesions due to the high risk of undiagnosed carcinoma [12]. Partial thickness excision has been described in the literature but it may be that the risks of treatment failure are significantly higher for this method of excision; this is an area for future research [3,8,13].
The main limitation in our study is the fact that we have not obtained data on those patients who underwent quadruple assessment and were judged to be unsuitable for local resection. Only data from local resection patients was added to the database so we did not have access to information for patients who pursued other treatment modalities.
In addition, we were unable to discover the ongoing management of 18% (n=6) of patients who received R1 resection and the longterm clinical outcomes this group as a whole was beyond the scope of this study. However, this is a substantial case series, we did not exclude any lesions based on size, position or previous local treatment.
Our analysis is based on intended depth of resection rather than actual depth however in over 96% of cases actual excision was of the intended depth. In order for patients to benefit from the quadruple assessment process the clinician must be able to reliably deliver the intended treatment [14, 35].
Shifting the focus of pre-operative imaging from T stage to predicting the appropriate plane of excision has enabled us to use transrectal ultrasound as part of a quadruple assessment process to accurately guide treatment decisions for patients with SPECC lesions. This allows the delivery of local treatment and offers the opportunity to deliver individualised treatment for a particular lesion. We are able to offer mucosectomy for patients with putatively benign or minimally invasive lesions with confidence. Full thickness excisions can be reserved for those with a deeper predicted depth of invasion. This enables us to minimise the procedural risks to which we expose our patients whilst achieving our treatment goal.
