Author(s): Leo G. Sapogin and Stanislav Konstantinov*
The article raises the question of the strange behavior of electrons in an atom, when the electronic orbitals of the P- and d-states of the atom have the form of eights with nodal points in the nucleus of the atom, as well as the discovery of the mysterious K-capture of an electron when the nuclei of atoms of some isotopes of chemical elements someh they sometimes capture an electron from the inner (K- or L-) electron shell of the atom. It has not been possible to explain these phenomena within the framework of the atomic model existing in quantum electrodynamics. In the new model of the atom, proposed by Professor Lev Sapogin in Unitary Quantum Theory, the electron makes quantum leaps within the orbital not randomly, as physicists thought, but through the nucleus of the atom, each time tunneling through it. In this case, the quantization of the energy levels (orbitals) of electrons in an atom is explained by the distribution of nodes and antinodes in a standing wave of an electron, and an integer number of de Broglie wavelengths should be located in the diameter of the electron orbital. The article shows the dependence of the magnitude of the interaction constants in the hydrogen nucleus and, in particular, the fine structure constant, discovered by the CMS cooperation in experiments at the Large Hadron Collider in 2019, during reactions in pp collisions with energies from 1 TeV to 13 TeV and an intranuclear pressure of 10³? Pascal. The value of the fine structure in the near-Earth medium and in a neutron star is given
Significant advances in quantum mechanics (especially under stationary conditions) began with a simple relationship between the de Broglie wavelength and the geometric properties of the potentials, which made it possible to solve the problem of the stability of atomic structures. Quantum bans are associated with an integer number of De Broglie wavelengths that must be placed in the length of a stable orbit. In this case, the particle was formally considered a point, otherwise it would be difficult to ascribe to the wave function the character of the probability amplitude. It is surprising that the abstract quantum ideology created by Niels Bohr, including the point principle and Bohr's principle of “complementarity”, which forbade even raising the question of the internal structure of elementary particles, turned out to be suitable for describing quantum reality. With the strict use of the new rules of the game, the researcher did not fall into any contradictions, and any paradoxes were eliminated by a simple prohibition to analyze them. However, today the quantum field theory within the accepted paradigm has exhausted itself. Physicists working in the framework of the Standard Model (SM) claim that all their predictions are confirmed experimentally. But this perfect (due to the lack of something better) model cannot even predict the masses of elementary particles, therefore the SM cannot be considered as the final theory of elementary particles. The Standard Model (SM) does not even have an algorithm for calculating the mass spectrum of elementary particles. CM contains from 20 to 60 freely adjustable parameters (there are different versions of CM) for calculating the mass of particles. All this strongly resembles the situation with Ptolemy's models of the solar system before the appearance of Kepler's laws and Newtonian mechanics. These earth-centered models of planetary motion in the solar system required first the introduction of so-called epicycles specially selected to coordinate theoretical predictions and observations. In addition, SM left some fundamental quantum questions unanswered, such as wave-particle duality, the nature of the Higgs Boson mass and explanations of the phenomena of chemical catalysis [1].
Professor Lev Sapogin’s Unitary Quantum Theory (UQT) breaks fresh ground in the theory of microcosmos, restoring the figurativeness and common sense excluded from physics by the Bohr’s antiquated complementarity principle [1, 2]. Within the framework of the Unitary Quantum Theory, Lev Sapogin succeeded in calculating the mass spectrum of all elementary particles without any correcting parameters [3]. Professor Lev Sapogin describes elementary particles as clumps (wave packets) of the field of the real world, which are identified with the polarizing, inhomogeneous cosmic environment of a quantum vacuum (dark matter) [4]. He writes: “Apparently, the mistake of all previous attempts to represent the particle as a wave packet was that the packet was built from de Broglie waves, which quickly spread out in a cosmic space. In the UQT, a packet is built of partial waves with a monstrously high frequency, the so-called Schrödinger jitter, and the de Broglie wave appears as a by-product, enveloping during the movement and evolution of the packet of partial waves into a real particle” [2].
In 1911, Ernest Rutherford, after doing a series of experiments, came to the conclusion that an atom is a semblance of a planetary system in which electrons move in orbits around a heavy positively charged nucleus located in the center of the atom (Rutherford's model of the atom) [5]. However, such a description of the atom came into conflict with classical electrodynamics. The fact is that, according to classical electrodynamics, an electron moving with centripetal acceleration must emit electromagnetic waves and, consequently, lose energy. Calculations showed that the time it takes for an electron in such an atom to fall to the nucleus is absolutely negligible. To explain the stability of atoms, Niels Bohr had to introduce postulates, which boiled down to the fact that an electron in an atom, being in some special energy states, does not emit energy (“the Bohr-Rutherford atom model”). Bohr's postulates showed that classical mechanics is not applicable to the description of the atom. Further study of the atom led to the creation of quantum mechanics, which made it possible to explain the vast majority of the observed facts. Bohr's postulates:
1. An atom can only be in special stationary, or quantum, states, each of which corresponds to a certain energy. In a stationary state, an atom does not emit electromagnetic waves.
2. An electron in an atom, without losing energy, moves along certain discrete circular orbits, for which the angular momentum is quantized:, where n is natural numbers, and h is Planck's constant. The stay of an electron in orbit determines the energy of these stationary states.
3. When an electron passes from an orbit (energy level) to an orbit, a quantum of energy is emitted or absorbed, where are the energy levels between which the transition takes place. When passing from the upper level to the lower one, the energy is emitted, when passing from the lower to the upper level, it is absorbed.
Using these postulates and laws of classical mechanics, Bohr proposed a model of the atom, now called Bohr's model of the atom [6] (Figure 1).
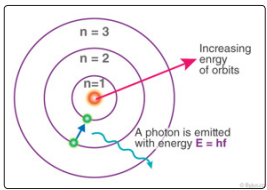
Figure 1: Bohr's Atom Model
Subsequently, Sommerfeld extended Bohr's theory to the case of elliptic orbits. It is called the Bohr-Sommerfeld model.
Later, in the De Broglie model, the atom began to be considered already as a nucleus around which standing waves are located, and the amplitude of the waves as the probability of finding an electron in a given place (Figure 2). Moreover, the motion of the electron was considered stable only when an integer number of standing waves fit along the orbit. At the same time, de Broglie waves make it possible not only to visualize the quantum structure of the field, but also to substantiate the regularity of filling the electron shells of an atom and the principle of periodicity of the periodic table.
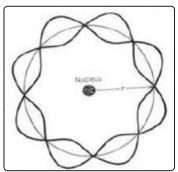
Figure 2: De Broglie's model of the atom
The potential field of an atom is a standing wave with a set of odd harmonics (1st, 3rd, 5th and 7th), the number of which in each set determines the principal quantum number n. The waves are distributed over the shells K, L, M, N, O, P, Q. Here each shell is an equipotential surface, and each half-wave has a quantum of electric charge (electron charge) and. field mass quantum (electron mass). In the probabilistic description of the electron as a standing wave or as an electron cloud, spin has no classical analogies. Thus, for a complete characterization of the state of an electron in an atom, four quantum numbers are required. The idea of the wave properties of the electron was developed in the works of Erwin Schrödinger, Paul Dirac, Werner Heisenberg and Max Born. This idea received experimental confirmation in 1927, when the phenomenon of electron diffraction was discovered. However, the discovery of the mysterious K-capture of an electron, when the atomic nuclei of some isotopes of chemical elements somehow sometimes capture an electron from the inner (K- or L-) electron shell of the atom, raised new questions. Within the framework of quantum mechanics, it turned out to be impossible to explain the mechanism of such capture of an electron by the atomic nucleus, and Lev Sapogin's Unitary Quantum Theory (UQT) made it possible to solve this problem. In Lev Sapogin's UKT, electrons inside an atom do not fly in orbits, as in Rutherford's model, but represent a standing electromagnetic wave that has no orbit and coordinates, but has a certain frequency and amplitude. This representation of the atom allows electron tunneling through the nucleus of the atom [2]. Lev Sapogin explained tunneling by the fact that the electric charge of an elementary particle is not constant in time, but periodically changes (oscillates) with a monstrously high frequency, the so-called Schrödinger jitter (“zitter-bewegung”), increasing to a maximum, then decreasing to zero according to a harmonic law. Therefore, quantum theory operates with time-averaged quantities of the effective charge of a particle and its mass, which also oscillates in time according to a harmonic law in the range from zero to a maximum [2]. For tunneling to occur, the particle must approach the potential barrier in the phase when the amplitude of the wave packet is small, and the particle, in the absence of charge, overcomes the barrier, "not noticing" it. At another stage, when the amplitude of the wave packet is large, nonlinear interaction begins, and the particle can be reflected from the barrier. From the point of view of the unitary quantum theory (UQT), Professor L. Sapogin, the motion of electrons in tunnel junctions can occur even at very low temperatures [2]. Thus, the quantization of the energy levels (orbitals) of electrons in an atom is explained by the distribution of nodes and antinodes in a standing wave, and an integer number of de Broglie wavelengths should be located in the diameter of the electron orbital. Professor Sapogin claims that being in the K-orbital closest to the nucleus of an atom, the electron makes quantum jumps within the orbital not randomly, as physicists thought, but through the nucleus of the atom, each time tunneling through it. It successfully tunnels due to the fact that at this moment it is in the “zero phase”, at which the instantaneous values of the charge and mass of the electron are close to zero, and therefore, by virtue of the law of conservation of momentum, at this time must develop a very high speed of movement through the nucleus atom. We believe that the proof of the correctness of this point of view is the fact that the electronic orbitals of the P- and d-states of the atom have the form of eights with nodal points in the nucleus of the atom (Figure 3).
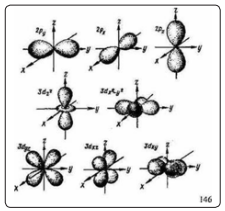
Figure 3: Forms of Electron Clouds for Different States of Electrons in Atoms
Since the regions allowed by quantum mechanics for the presence of an electron in them are only the inner regions of these orbitals, then in order to get from one half-branch of the “figure of eight” to the opposite, an electron must slip through the atomic nucleus. This allows us to take a fresh look at the mechanism of the mysterious K-capture of an electron in an atom. As is known, electron capture consists in the fact that the nuclei of atoms of some isotopes of chemical elements in some mysterious way sometimes capture an electron from the inner (K- or L-) electron shell of the atom. Physicists have long been tormented by the question of how such a capture is accomplished if the electron in the atom, according to existing concepts, is very far (on nuclear scales) from the nucleus. But if an electron, according to Sapogin, constantly tunnels through the nucleus of an atom, then everything becomes clear. After all, any accidental fluctuation in the motion of an electron or nucleus can disrupt tunneling, and then the electron is either captured by the nucleus, or nonlinear interaction begins, and the particle can be reflected from the barrier. In this case, not the entire electron is captured, but only its electric charge and most of the mass, which are attached to one of the positively charged protons P of the nucleus, which turns into a neutron N, the mass of which is greater than the mass of the proton. But the remainder of the electron in the form of an electron neutrino ν? flies out far beyond the atom. Physicists assume that in this case a process is taking place in the nucleus of an atom:

Which, however, has never been observed in experiments on the bombardment of protons by beams of accelerated electrons [7].
As a result of K-capture, the total positive charge of the nucleus decreases by one (in units of the proton charge). Therefore, the nucleus during K-capture is transformed into the nucleus of an atom of one of the isotopes of a chemical element, which is in front of the original chemical element in the periodic table. True, the nuclei of atoms by no means all isotopes can undergo such a transformation. It is realized only when the selection rules and conservation laws existing in nuclear physics are fulfilled. In particular, the sum of the masses of the original nucleus and the electron must be greater than the mass of the resulting atomic nucleus. The proof of the correctness of our understanding of electron capture is the presence of the phenomenon of internal conversion of electrons in the atom. It consists in the fact that when the selection rules prohibit the emission of a γ-quantum by an excited nucleus of an atom, then the excitation is most often removed due to the transfer of the excitation energy of the nucleus to the electron of the atomic shell. The transferred energy is so high (up to MeV) that tens of electrons are knocked out of the atom. Until now, the mechanism of transfer of excitation from the nucleus to the electron of the atomic shell has been a mystery to physicists. Earlier, it was mistakenly believed that excitation to an electron is transmitted by a γ-ray emitted by the nucleus, but it turned out that such radiation is prohibited by the existing selection rules. Therefore, it remains only to assume that the excitation from the nucleus to the electron of the atomic shell is transmitted when, in accordance with Lev Sapogin's UQT, this electron penetrates the nucleus of the atom.
The solution of the basic nonlinear equation of the UQT allowed Lev Sapogin to theoretically calculate the elementary electric charge and the value of the fine structure constant α with high accuracy. Sapogin has α = 1 / 137.962, and the experimentally obtained value is α = 1 / 137.03552 [3]. The magnitude of the fine structure constant, α, was introduced into physics in the early 20th century by Arnold Sommerfeld to describe the energy sublevels found experimentally in the emission spectra of atoms. Since then, many other manifestations of the same constant connection have been found in various phenomena related to the interaction of elementary particles. In quantum electrodynamics, the fine structure constant is a measure of electromagnetism - one of the four fundamental forces in nature (the others are gravity, weak nuclear force, and powerful nuclear force). The electromagnetic force keeps the electrons moving around the nucleus in the atom of the universe, otherwise all matter would be shattered into pieces. Currently, in quantum electrodynamics, the following value of the fine structure of elementary particles has been experimentally obtained:
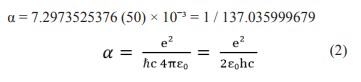
Where e is the elementary electric charge, ? = h / 2π is the Dirac constant (or the reduced Planck constant), c is the speed of light in vacuum, ε0 is the dielectric constant.
Until recently, it was believed that this is an invariable force in time and space. However, recent experiments make it possible to detect differences in the meaning of the fine structure as one of the fundamental constants in the space of the late Universe and in the course of its evolutionary development [8]. A team from the University of New South Wales under the guidance of Professor John Webb, the University of Technology at Swinburne and the University of Cambridge presented on a report on the detection of changes in the fine structure constants α. The CMS collaboration in experiments at the Large Hadron Collider in 2019 studied the distribution of reaction products in pp collisions with energies from 1 TeV to 13 TeV. It was found that a decrease in the mass of elementary particles obtained from data up to an energy of 13 TeV, as well as a decrease in the value of the interaction constants at a confidence level of 95% depend on the energy at which the measurements were made. This effect, explained by the polarization of the vacuum, was actually observed in experiments, in particular, a decrease in the mass of b- and c-quarks, as well as a decrease in the strong interaction constant, were measured [9]. The vacuum polarization effect leads to charge screening at low energies. With increasing energy, fine structure magnitude (α) changing logarithmically:
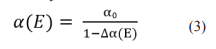
Where E is the electric field strength, ? α is the incremental value is calculated as part of QCD
In 2018. Professor Volker Burkert carried out a series of experiments at the CEBAF accelerator. After the collision of fast electrons with the mass of liquid hydrogen (the source of protons), the researchers registered the particles arising from their interaction - an electron, a proton and two photons. This allowed for the first time to measure the pressure at the center of the proton, bombarding the proton with electrons, the energy of which reached 100 MeV or more, which allowed the electron to penetrate into the structure of the proton (Fig.4) [7].
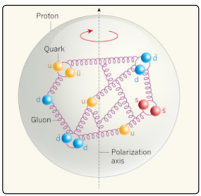
Figure 4: The structure of the proton, quarks and gluons
Volker Berkert and his colleagues from Jefferson's laboratory found that the pressure in a proton can exceed 10³5 Pascal [7]. It is known that at such a pressure polarization of the quantum vacuum and a change in the interaction constants, including the fine structure constant, are observed [8]. The value of the fine structure under terrestrial conditions (at normal pressure and temperature) is equal to α? = 0.0072975, and the elastic deformation force F = 1.155 x 10¹9 [kg / s²] is determined by electromagnetism in the theory of quantum electrodynamics (QED). However, inside the proton nucleus, where the theory of quantum chromodynamics (formula 3) and the experiments of Professor Volker Berkert predict the elastic deformation value F = 5.211 x 10²6 [kg / s²], under the influence of nuclear forces, the value of the fine structure can reach a greater value than under the influence of the forces of electromagnetism [7].
In an article by astrophysicists from Finland, published on June 1, 2020, it is said that “the matter inside the most massive stable neutron stars is interpreted as evidence of the presence of quark matter nuclei, in which the speed of sound almost reaches the speed of light” [10]. It is believed that a form of this strange substance, called quark-gluon plasma, filled the newborn universe about 20 microseconds after the Big Bang. It behaved like an extremely hot liquid, which then cooled to the state of "ordinary" matter that fills the universe today. Currently, the only place in the universe where quark matter can still be found is at the epicenter of particle collisions at the Large Hadron Collider and possibly the heart of a neutron star [10].
The new physics, based on the Professor Lev Sapogin’s Unitary Quantum Theory , rejecting the point principle and the principle of “complementarity” of Bohr, who forbade even raising the question of the internal structure of elementary particles, made it possible to propose a modern model of the atom and take a new approach to solving the problem of cold nuclear fusion [11].
