Author(s): Arturo Solís Herrera and Oscar Aguilera Madrigal
Life originated in anoxia, but paradoxically many organisms came to depend upon oxygen for survival, independently evolving diverse respiratory systems for expel CO2 and acquiring oxygen to and from the environment, respectively. Thereby, Oxygen, a vital gas, and a lethal toxin, represents a trade-off with which all organisms have had a conflicted relationship.
The study of oxygen movements in the tissues of the human body has been a matter of great interest ever since centuries. In the beginning of the past century, Dr. Christian Bohr and August Krogh’s work on respiratory physiology and capillary modelling using mathematical models to calculate molecular transport in microcirculation, trying to determine the negative impact of lack of oxygen transport to tissues. Supposedly, computer simulation allowed investigation of the dynamic and non-linear characteristics of the systems. But the results have been and are contradictory.
In Germany, Dietrich Werner Lubbers (1917-2005) obtained several patents related to designs for the study of gases in tissues. The aim of Dietrich Lübbers’ research was to understand the entire pathway and regulation of oxygen transport from the blood into the mitochondria. Assessment of pO2 histograms on most organs, revealed a remarkable similarity under physiological conditions: a Gaussian distribution always with less than 5% of values less than 5mmHg.
Other studies detected changes in the concentrations of oxy- ([HbO2]) deoxy- ([HHb]) and total haemoglobin ([HbT]=[HbO2]þ [HHb]) measured using near infrared spectroscopy (NIRS) [1]. It has been shown that diabetic rats have markedly decreased oxygen availability in the kidney, supposedly resulting from increased oxygen consumption [2].
Oxygen transported to tissue, after reaching the tissue microcirculation, diffuses from the blood plasma through the walls of the micro-vessels into the interstitial (pericellular) space and then from interstitial space into the cells and finally to the mitochondria. As it diffuses, from the source (blood plasma) to a sink (mitochondria), an oxygen pressure gradient is formed in which the pressure is lower at the sink than at the source. The difference in oxygen pressure between the blood plasma and the mitochondria increases with increase in the rate of oxygen consumption by the mitochondria and the distance from the vessel to the mitochondria. The distance over which oxygen can be supplied to the mitochondria is, therefore, determined by a) the rate of oxygen consumption by the mitochondria, b) the distance from the blood plasma (the oxygen source) to the mitochondria and c) the oxygen pressure in the blood plasma. (Figure 1).
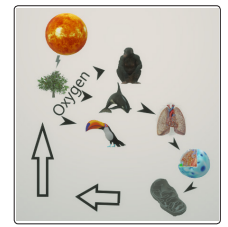
Figure 1) Scheme that represents traditional concepts about the relatively simple flow of oxygen since it is produced in plants, thanks to the energy of the sun, then is delivered into the atmosphere by the leaves of the trees, later is inhaled by the different species that have lungs, where it is absorbed into the general circulation by the pulmonary tissues to be distributed to all cells of the body where its final destination is the mitochondria where it plays the role of electron acceptor , and is then expelled in theform of CO 2 (arrows), to be absorbed by plants, which transform it into carbohydrates, with glucose being the best known exponent and the most water-soluble.
Although the prevailing concepts about the generation and flow of oxygen in biology seem as firm as it is simple, several mysteries could not be explained that make this type of scheme be theoretical for the most part.
One of them is the false belief that the leaves of plants expel oxygen into the atmosphere for the benefit of living beings, who absorb it from the atmosphere so that the cells that make up them combine it with glucose; thus obtaining the energy that every living being requires to drive the thousands of chemical reactions that happen incessantly within organisms. But we forget about oxygen toxicity, which is the main reason why plants throw it into the atmosphere, because otherwise oxygen levels would rise dangerously by de-naturating the compounds it contains and rapidly inactivating the fundamental property of chlorophyll which is the irreversibly dissociation of the water molecule as follows:
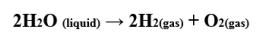
It is surprising that chlorophyll carries out a reaction of such importance and accuracy at room temperature, because in the laboratory it is necessary to heat the water two thousand degrees Celsius so that the liquid water becomes its gaseous components. The fact that the union of two relatively stable gases such as hydrogen and oxygen form a liquid compound is another wonder of the mysterious compound, we call water. And it is explained by the action of hydrogen bridges. Something unique in nature.
Another mystery that makes the traditional scheme of oxygen generation and flow in nature unlikely is that gases are almost not combined with water. Oxygen is a non-polar molecule and water is a polar molecule, therefore they are not and cannot combine. Therefore, the oxygen does not dissolve in the water. Non-polar molecules are even known to be hydrophobic, i.e. they repel water.
Several explanations have been proposed, trying to understand as that the oxygen inside the body comes from the atmosphere. For instance, the random mixing of water. Some oxygen molecules will happen to find their way into water and not find their way out for a while. Other theory is that oxygen is not entirely nonpolar; it lacks a permanent dipole moment, but it is polarizable. For instance, oxygen is a gas at room temperature but can be cooled into liquid state, which means something must be keeping them together.
The very snugly solubility of oxygen (264 μM at 25 °C) increases proportionally increased partial oxygen pressure. But the increase in temperature decreases the solubility of oxygen in water.
The higher the temperature, the faster the molecules of oxygen are moving, and the easier it becomes for the oxygen molecules to escape the water’s surface. Consequently, increasing temperature causes oxygen to become less soluble, and oxygen is more soluble in cold water.
High humidity reduces the partial pressure of the oxygen in the air very slightly, so high humidity reduces oxygen solubility. Sometimes water may be oxygen-poor for reasons having little to do with the solubility. During algae blooms, for example, bacteria decomposing the dead algae use up most of the available oxygen in the water, creating an oxygen-poor environment. In a situation like this, the problem is not that the oxygen is less soluble per se --- rather, that it is being consumed rapidly by the decomposers in the ecosystem.
At a temperature of 37°C, salinity of 1.5 and at a pressure of 1 atmosphere (760 mm Hg), the solubility of oxygen is: 209.51823199476186 μM, 6.704583423832379 mg/l, and 5.332429456339571 ml/l.
For comparison aims, pure iron has an oxygen solubility of about 2300 ppm (0.23 wt.%) at 1600 °C. The solubility of CO2 in water 3.4 g gas per kg water, at 0 °C; and a at 37 °C is 1.0 g gas per kg water.
The solubility of Hydrogen (H2 ) in water at 0°C is 0.0019 g gas per kg water, and at 37 °C is 0.0014 g gas per kg water. The solubility of Nitrogen (N 2 ) in water at 0°C 0.03 g gas per kg water, and at 37 °C 0.013 g gas per kg water. The solubility of Oxygen (O2 ) in water at 0 °C is 0.07 g gas per kg water, and at 37 °C is 0.033 g gas per kg water.
Current dogma is as follows: Oxygen: Essential to sustain life. Key function: Inhaled oxygen is absorbed by blood; dissolved oxygen reacts with food (present in the body as sugars) to produce energy and heat - metabolism. Waste products of metabolism of oxygen - carbon dioxide and water.
However, if we look carefully at the physical and chemical properties of oxygen, as well as the composition of the tissues that make up the pulmonary alveoli, it would appear that everything is against the absorption of oxygen from the air by the lungs.
| Substance | Inhaled air (%) | Exhaled air (%) |
|---|---|---|
| Nitrogen | 78 | 71 |
| Oxygen | 20.9 | 61 |
| Argon | 0.9 | 0.9 |
| Carbon Dioxide | 0.0390 | 4.0 |
| Water vapor | Variable | Variable |
Table 1) Air weights 0.0012929 gram per cubic centimeter or 1.2929 kilogram per cubic meter. It is doubtful that 4.9% oxygen is absorbed, because we must consider that the volume of CO 2 increased a hundred times in the exhaled air, from 0.0390 to 4.0 %.
However, differences in the quality and composition of air depends on population of location, activity of the habitants, activities in the neighboring regions, local weather patterns, geographical features of location. Calculation of effects on body, lung, and blood physiology based on concentration of substance or element in air, length of time, rate of breathing.
Atmosphere is 21 % of Oxygen, thereby, there are 21 oxygen molecules per 100 molecules in air, or 21 L of oxygen per 100 L of air (parts per hundred -percent-); or 21 0000 L per 1 000000 L of air (parts per million).
The atmospheric concentration of O2 has remained constant for several hundred years at 20.95%. This means that exactly 20.95% of the molecules in the atmosphere are O2 molecules. The percentage is the same on the beach in Florida and in the mountainsof Logan, Utah. However, the absolute O2 concentration, or the amount of O2 molecules per unit volume, does not remain constant. An O2 tank is required to climb Mount Everest, even though the relative O2 concentration is still 20.95%.
The absolute O2 concentration changes a few percent from day to day with changes in barometric pressure and temperature. As barometric pressure decreases, or as temperature increases, air expands and the number of O2 molecules per unit volume decreases. The opposite occurs as barometric pressure increases or as temperature decreases.
The absolute O2 concentration determines the rate of most biological and chemical processes, thereby, the relative O2 concentration is typically reported. Although the above is theoretical, as pulmonary alveoli does not have the ability to absorb oxygen in sufficient amounts to saturate hemoglobin, since hemoglobin per gram contain 1.36 to 1.5 milliliters of oxygen, much less to cover the liters and liters of oxygen that the body would require if the energy it uses for its metabolism came from the oxidation (combustion) of glucose.
Normally, oxygen solubility is strongly dependent on the amount of dissolved electrolyte salt(s) (decreases at higher concentration of electrolyte), temperature (decreases at higher temperatures), and pressure (increases at higher pressure) [1,2,3]. It seems that oxygen has everything against it so that it can enter the human body through the lungs. It seems that oxygen has everything against it so that it can enter the human body through the lungs, then be transported by the bloodstream, and finally pass through the at least two bilipid membranes: the cell membrane and the mitochondrial membrane that double. Oxygen is heroic trying to get from the atmosphere to the mitochondria, and worst of all it is that no biologic mechanism is known to increase its diffusion or at least its solubility, despite the millions of years of evolution. Then nature most likely optimizes its oxygen natural hydrophobia (non-polar) to keep it out of the body, as oxygen is generated at the same time as hydrogen when the water molecule is dissociated.
Hemoglobin is a metalloprotein found in the blood cells of vertebrates and in some non-vertebrate organisms. Each hemoglobin molecule can carry up to four molecules of O 2 that is one oxygen molecule for each of the four hemoglobin subunits. But if we reflect on the oxygen, a non-polar molecule is hydrophobic, and once it entering in the respiratory pathways and reaching the alveoli, it tends to get away from the respiratory tissues since the water is present inside the cells and over the air conducting and absorbing surfaces which are characteristically covered with water in diverse forms. By other hand, exists the real possibility that hemoglobin, given its marked resemblance to chlorophyll, may be able to irreversibly dissociate the molecule from water. A blood Oxygen level below sixty mm Hg is considered low. The average partial pressure of oxygen in the atmosphere in mm Hg is 160 mm Hg.
For a normal adult male the oxygen content of arterial blood can be calculated: Given arterial oxygen saturation (SpO2 ) = 100%, Hb = 15 g/100 ml and arterial partial pressure of oxygen (PaO2 ) = 13.3 kPa, then the oxygen content of arterial blood (CaO2 ) is: CaO2 = 20.1 +0.3 = 20.4 ml/100 ml. Similarly, the oxygen content of mixed venous blood can be calculated. Given normal values of mixed venous oxygen saturation (SvO2 ) = 75% and venous partial pressure of oxygen (PvO2 ) = 6 kPa, so: CvO2 = 15.2 + 0.1 = 15.2 ml/100 ml. The theoretical maximum oxygen carrying capacity is 1.39 ml O2 /g Hb, but direct measurement gives a capacity of 1.34 ml O2 /g Hb. 1.34 is also known as Hufner’s constant.
Tidal volume is the amount of air breathed in during a normal
breath. In a healthy, young human adult, tidal volume is approximately 500 mL per inspiration or 7 mL/kg of body-mass. Air
weights 0.0012929 gram per cubic centimeter, thereby 500 cc of
air weights 0.645 grams.
Oxygen delivery is the amount of oxygen delivered to the peripheral tissue and is obtained by multiplying the arterial oxygen
content (CaO2
) by the cardiac output (Q). For CaO2
= 20.1 ml/100
ml and Q = 5 l/min:
Oxygen delivery (DO 2 ) = 1005 ml/min
The oxygen returned is given by the product of the mixed venous oxygen content (CvO2 ) and the cardiac output. For CvO2 = 15.2 ml/100 ml and Q = 5.0 l/min: Oxygen return = 760 ml/min
Oxygen uptake is the amount of oxygen taken up by the tissues that can be calculated from the difference between oxygen delivery and the oxygen returned to the lungs in the mixed venous blood. Thus, Oxygen uptake (VO 2 ) = (oxygen delivery) - (oxygen return) = 1005 - 760 = 245 ml/min
The primary goal of the cardio respiratory system is to deliver adequate oxygen to the tissues to meet their metabolic requirements, a balance between VO 2 and DO 2 . The balance between oxygen uptake by the body tissues and oxygen delivery to them is assessed by:
The oxygen content of mixed venous blood CvO2 , which is normally about 15 ml/100 ml. The extraction ratio, which is the ratio of VO2 to DO2 expressed as a percentage. Normally the extraction ratio is about 25% but can double to 50% if tissue demand increases. However, this goes against the observation of Nature as no mechanism has been identified that increases oxygen absorption in tissues or cells, and on the other hand hypoxemia does not act as a stimulus in the respiratory center.
Both above indices are dependent on mixed venous saturation (SvO 2 ), and cardiac output. Theoretically, if the level of hemoglobin is halved, the oxygen content of arterial blood will be halved. But it is theoretical, because in patients it is not so linear.
Oxygen absorption into the lungs is extremely limited by the physical and chemical properties of both oxygen itself and the characteristics of alveoli tissues. Moreover, if there are not-known mechanisms that increase oxygen absorption, then we have the explanation why tachycardia does not significantly increase oxygen levels in the blood.
The human lung consumes about 5-6 ml oxygen per minute at an esophageal temperature of 28 degrees C. Pre-bypass whole-body oxygen consumption measured at nearly normothermic conditions was 198 +/- 28 ml/min. Mean lung and whole-body respiratory quotients were similar (0.84 and 0.77, respectively). Extrapolating lung oxygen consumption to 36 degrees C suggests that the lung consumes about 11 ml/min or about 5% of total body oxygen consumption [3]
The lung fulfills specialized and energy-consuming functions including tracheobronchial clearance, regulation of distribution of air and blood flow, and surfactant turnover. Therefore, the constituent cells of the lung have metabolic requirements that must be satisfied to maintain functional and structural integrity. This amount may be increased markedly under pathologic conditions such as lung infection or adult respiratory distress syndrome [4]. Although it is not really that it increases oxygen consumption to obtain more energy, since oxygen has so many locks to penetrate the body, that its availability is extremely limited and irregular. What the body requires is hydrogen, which is obtained at the same time as oxygen by dissociation of water. Living beings do not take oxygen from the air, and the example is trees, because the oxygen they expel into the atmosphere due to their toxicity does not obtain it from the atmosphere itself, but from the water they contain; and the human body works in a similar way
Any tissue in the human body in which hydrogen levels decrease will show first functional and then anatomical alterations since it does not have enough energy to carry out its functions and to preserve the shape. Well, oxygen levels function as an indirect indicator of hydrogen levels since the body gets both molecules at the same time when it dissociates water. When we detect hypoxia in the tissues, we must think that the hydrogen is the one that is missing. By supplying hydrogen, with its precious energy load and powerful antioxidant effect, the tissues correct their function and, where appropriate, even form, as we require energy even to preserve the shape. If we must always keep in mind that cells, tissues, organs, systems, and the human body in general use energy in many ways.
Returning to the lungs: In the normal lung, total bronchial blood flow is estimated to be about 1% of cardiac output, contributing a small amount to pulmonary capillary blood flow and gas exchange [5]. The major portion of this blood supplies the bronchial walls and the visceral pleura and is drained into the bronchial veins. We believe that the alveolar walls derive oxygen chiefly from the alveolar air, whereas the bronchi, the smaller air passages, and major portions of the visceral pleura use oxygen carried by the bronchial flow [6].
An interesting point to consider relates to trans-pleural diffusion. In isolated nonperfused dog lungs, measurable quantities of oxygen and carbon dioxide can traverse the visceral pleura. The absolute quantity varies with the magnitude of the concentration gradients. This can be derived from experiments in which the concentration gradient between extra-pleural and inspired gas was increased from 6% to 12%, resulting in a parallel doubling of gas transfer [7]. Oxygen has many impediments to entering the lungs, trans-pleural oxygen levels should be interpreted because of the dissociation of water in the surrounding cells, without as oxygen coming from the pulmonary alveoli. Oxygen is a poisonous gas, and for centuries it is known that poisons in small doses, tend to awaken positive reactions from the body. Hence, when we double the levels of oxygen administered, the body reacts by elevating the dissociation of the water. An effect like homeopathy. The lungs in situ are completely covered by tissue, limiting markedly gas exchange with ambient air.
Breathing is a fundamental function in life. Traditionally the concept is that the air we breathe in has precious oxygen that fuels the breakdown of sugars and fat in our cells. However, our discovery of melanin’s intrinsic property of dissociating the water molecule breaks down the sacrosanct role of glucose as an energy source into a thousand pieces; so, the supposed fundamental role of oxygen simply fades.
Carbon dioxide is a byproduct of sugar and fat breakdown in cells, but the purpose of glucose metabolism is for cells to have the building blocks needed to replenish worn molecules, and CO2 , the most oxidized form of carbon, needs to be removed from our body. The blood acts as a transport medium. Carbon dioxide diffuses out of cells and is transported in blood in a few different ways: less than 10% dissolves in the blood plasma, about 20% binds to hemoglobin, while the majority of it (70%) is converted to carbonic acid to be carried to the lungs through the water found in the bloodstream. but since gases are hardly combined with water, carbon dioxide needs to be modified in such a way as to substantially increase its solubility in water, for example by transforming it into carbonic acid which, in turn, spontaneously transforms into bicarbonate.
It is a remarkable fact that the same enzyme that allows carbon dioxide to quickly exit cells when is solubilized from the gaseous state by transforming it into carbonic acid, therefore, it is the same compound that allows the rapid exit of the carbon dioxide from the water contained in the bloodstream to the pulmonary alveoli, since the action of said enzyme is reversible and thereby re-composes the carbonic acid or bicarbonate in gaseous carbon dioxide , which is easily diffused into the air contained in the lung spaces are upholstered with the enzyme.
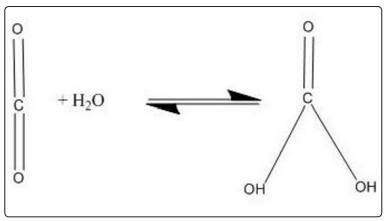
Figure 2) Action of carbonic anhydrase. The enzyme presents also in red blood cells, carbonic anhydrase, aids in the conversion of carbon dioxide to carbonic acid and bicarbonate ions. When red blood cells reach the lungs, the same enzyme helps to convert the bicarbonate ions back to carbon dioxide, which we breathe out. Although these reactions can occur even without the enzyme, carbonic anhydrase can increase the rate of these conversions up to a million-fold.
The lung raises the CO 2 concentration of alveolar air expired about 100 times, from 0.039 % to 4.00%. And it probably does not rise any higher because that figure is the upper limit of CO2 solubility in aqueous solutions. But CO2 is also a non-polar gas that is hardly mixed with water. And interestingly, nature did develop a solution to increase its solubility. Carbonic anhydrase.
It is paradoxical that we continue to believe that the exchange of gases that takes place in the lungs will mainly be oxygen when nature does not move a finger to increase its solubility and absorption. Instead for the exchange of CO 2 , nature developed an enzyme that significantly increases solubility, and the most interesting thing is that it is the same enzyme in all living beings, which was to be expected since we have a common origin
Carbonic anhydrase is an enzyme that solubilizes CO2 by transforming it into carbonic acid, which is highly soluble in water,so when CO 2 comes out of cells in the form of gas, carbonic anhydrase solubilizes it to be easily transported through the bloodstream into the lungs.
But because it is an enzyme whose effect is reversible, when carbonic acid (H2 CO3 ) or bicarbonate (HCO3 - ) reaches the lungs, its action becomes reversible and then begins to transform the bicarbonate into CO2 into a gaseous state so that it can be efficiently expelled by the pulmonary alveoli.
The enzyme carbonic anhydrase is extremely efficient; its pKa (constant acid dissociation) is one of the highest known: between one and three million molecules per second, which tells us about the great importance of this enzyme in biology.
It is wrongly believed that Plants use oxygen for producing energy, and release carbon dioxide, however, a double energy system is a luxury that even Nature cannot afford. The plants use glucose just as we do, as a universal precursor to the molecules or organic compounds that make up them, so that they also expel relatively small amounts of CO 2 , which is explainable by the metabolic differences between plants and humans, where the need to expel CO2 is more demanding because they produce more, for example during movement, during exercise, and we cannot recycle it by forming new carbohydrates, which plants can do perfectly. Green plants can convert water and carbon dioxide into sugars in the presence of sunlight. This process, photosynthesis, uses carbon dioxide from the atmosphere. Gaseous carbon dioxide is stored in plants as bicarbonate ions. In both land and water plants, carbonic anhydrase plays a role in converting bicarbonate ions back to carbon dioxide for photosynthesis. Another interesting biological phenomenon where this enzyme plays a role is the calcification of corals. Seawater calcium reacts with the bicarbonate produced by carbonic anhydrase from the coral polyps, forming calcium carbonate. This is deposited as the hard exterior of coral.
Human beings released an estimate of 700 grams of carbon dioxide a day. Oppositely, the absorption of CO 2 by the trees varies according to the age and type of plant, but we could say that it absorbs an average of 33 grams (26 pounds) of carbon dioxide per day. In addition to plants masterfully processing CO2 , they also have a surprising ability to store it. That is why the amount of carbon dioxide that plants expel is negligible. That is why plants do not have a system that could be equated to the pulmonary in humans and other species, because they produce notably less carbon dioxide, transform it into glucose in a very efficient way, and are able to store it. This is so true that plants absorb a significantly higher amount of CO2 than they expel.
Going back to carbonic anhydrase, it is surprising how much literature we can find about it in research journals, what was to be expected given its important role in biology however such literature is silent in relation to the energy source it uses to carry out its function [8].
As in any chemical reaction, the action of carbonic anhydrase requires energy which it uses in several ways, for example: the essential activation energy. The presence of these ubiquitous enzymes in so many tissues and in so different isoforms tells us that its energy source has similar characteristics, that is: it is also ubiquitous and is throughout the phylogenetic tree, and melanin meets the requirements.
So, when the generation and distribution of energy from melanin is disturbed by the action of contaminated water, polluted air, pesticides, herbicides, fertilizers, industrial wastes, solvents, daily emotional stresses, etc., and when a process as accurate as the dissociation of the water molecule loss the balance, the energy output is modified and the chemical reactions that depend on it, tend to disorganize.
If the energy that comes from melanin fails, the function of carbonic anhydrase is also disturbed, and in case of the lung the patient will feel that he is short of air, but not due to lack of oxygen, but by excess CO 2 . In cases where the generation and distribution of melanin energy is significantly altered, as in COVID-19, symptomatology is very congruent, because melanin, for one reason or another, is dissociating the water molecule little, which we can detect by decreasing oxygen levels in the blood (less of 90 % or 60 mm Hg), because the oxygen that our body contains extracts it from the water inside it , not from the atmosphere.
Hence the logic of the sequence of clinical events in COVID-19 patients: first is hypoxia that reflects energy failure, and then else. Dyspnea is because the body cannot expel the CO 2 that the body produces properly, and the patient feels as if it is underwater. This is explained because carbonic anhydrase does not have enough energy to perform its fundamental function.
The series of events that follow hypoxemia in COVID-19 patients can be explained in a similar way, since on the one hand, we have the toxic effects of elevated CO 2 levels, which are in case diffuse, and on the other hand we have that all other systems also begin to malfunction because they also depend on the generation and distribution of energy from melanin. And the deficiency in energy explains the so-called“;happy hypoxemia”;, in which the sick, despite having alarming oxygen levels up to 50%, do not seem to be greatly affected, but even the processes through which CO2 are produced in the body, just as if it is a waste product, also require energy.
It is not by chance that COVID patients who are intubated have a fatality rate of more than 90%, and it was to be expected since the lungs are not the natural route of oxygen into the body. Therefore, the human organism feels assaulted and thereby worsens the multiple imbalances already present, which eventually leads to a fatal outcome.
Hypoxia is not even the major source of lactate nor does added oxygen necessarily enhance its metabolism. Authoritative investigators have now agreed that lactate, long thought to be merely the end-product of hypoxia, has far greater significance. In fact, lactate has many aerobic sources including aerobic glycolysis, and activated leukocytes, and performs many important and previously unanticipated functions [9].
It is interesting that fermentation, defined as chemical process by which molecules such as glucose are broken down anaerobically, used at least 10 000 years old in manufacture of wine and beer; it also occurs in muscle fiber, in embryonic and in cancer cells. Fermentation reactions are not peculiar to the action of yeast but also occur in many other instances of glucose utilization, where in oxygen absence, glucose is reduced to lactic acid, alcohol, glycerol, butyl alcohol, acetone, monosodium glutamate, citric acid, gluconic acid, and of course carbon dioxide.
Glucose is an essential provider of the building blocks that mold up mammalian life, being the end-product lactate or, upon full oxidation of glucose via respiration in the mitochondria CO 2 . Intumors, and other proliferating or developing cells, the rate of glucose uptake dramatically increases, and lactate is produced, even in the presence of oxygen and fully functioning mitochondria [10].
This process known as Warburg effect, has been documented for over 90 years and extensively studied over the past 10 years with thousands of papers reporting to have established either its causes or its functions. Despite this intense interest, the function of the Warburg Effect remains unclear.
The rate of glucose metabolism through aerobic glycolysis is higher such that the production of lactate from glucose occurs 10-100 times faster than the complete oxidation of glucose in the mitochondria [11]. By discarding glucose as an energy source and focusing on its role as a source of carbon chains, we may think that fast-growing tissues require as many organic molecule precursors as possible with the lowest production of toxic products such as CO 2 and ATP. That is why CO 2 production is not significantly increased in pregnant women, children during the growth stage, or even in cancer patients.
For us, the function of ATP is temperature regulation, because when ATP is hydrolyzed, energy is absorbed, and when re-formed from ADP, energy is released. which goes according to the evolution of the temperature that we observe in fermentation. Temperatures are consistently lower than the temperatures observed during mitochondrial respiration. Briefly, we have found a correlation with the temperature control of cells, tissues, and the body in general and the number of mitochondria and therefore the amount of ATP. The greater the number of mitochondria, the greater the need for temperature control for one reason or another.
So, it is not uncommon for researchers, after 90 years, to be unable to decipher the foundations of the Warburg effect, since they are looking where there is nowhere, because neither mitochondria nor ATP are sources of energy.
It is confusing that Muscle O 2 consumption (UO 2 m) during a fast-transient change (e.g., exercise) cannot be directly evaluated by measuring oxygen uptake in the lungs (VO 2 p) [12]. The dynamics of the oxygen concentration in blood depend on the spatial distribution and temporal variation of the variables such as blood flow and hemoglobin oxygen saturation that affect convective and diffusive transport of oxygen in the microcirculation. However, these effects are not directly measurable during muscle contraction [13].
The binding and buffering of O2 and CO2 in the blood influence their exchange in lung and tissues and their transport through the circulation. Trying to investigate the binding and buffering effects, different models of blood-tissue gas exchange were used. The models account for hemoglobin saturation, the simultaneous binding of O2 , CO2 , H+ , 2,3-DPG to hemoglobin, and temperature effects [14]. However, results are far from being useful.
There exist many constraints to studying intact lung metabolism and there have been numerous attempts to develop a mathematical approach to understanding lung gas interchange through modeling technologies based on in vivo and in vitro data. Most of the current literature on modeling in the lung and other tissues like brain, does not consider, the possible competition by other fuels at the transport level or the feedback or even redox mechanisms
Despite the hard work of different researchers to develop mathematical models that accurately simulate the functioning of the different organs of the body; the results have been less than mediocre. The main reasons is that these faulty models are because they are based on false premises such as that glucose as an energy source and that our body obtains oxygen from the atmosphere, introducing it through the lungs to finally transport it through the bloodstream to the different tissues and cells of the body, where they combine with glucose since, theoretically; glucose oxidation (combustion) is the way the body gets the much-needed energy it requires for its metabolism.
Glucose metabolism is described differently in the different biochemistry textbooks. The reason is because more than 95% of the biochemical logic proposed to try to explain theoretical processes, are precisely theoretical so each author ends up describing them in their own way [15].
Glucose dogma as an energy source is deeply rooted. Glucose is the universal precursor to any organic matter in plants and animals. But glucose cannot provide the energy your own metabolism requires. If so, diabetics would fly
The history of glucose begins because every form of life on earth depends on the light coming from the sun. And it is also considered dogmatic that the main biological molecule that can transform sunlight into energy that can be used by living beings is chlorophyll, which transforms photonic or luminous energy into chemical energy by irreversible dissociating the water molecule, expelling oxygen to atmosphere, supposedly in benefit of other species, as human beings, whom take this oxygen from atmosphere, introducing into the organism through a diffusion-driven gas interchange in the lungs.
However, since 1897, John Scott Haldane, published that diffusion could not explain the different bloody concentrations of oxygen that could be experimentally sized, and not only in humans but in different species [16].
Our circumstantial discovery of the intrinsic property of melanin is a disruptive finding about biology in humans, modifying it in its entirety. But it will allow us to correct many mistakes inherited from the past which will result in the quality of life of the sick [17].
It is now possible to concatenate the flow of energy and mass into biological processes, knowledge that now becomes urgent to apply in view of the deterioration of the environment and manifests itself by diseases that we could not fully understand such as COVID-19 and others.
This is a key moment to rescue the sick and to move forward, because the new biology that is developing from the knowledge that photosynthesis also occurs in humans, makes us more aware of the urgent need to protect the environment, otherwise the next pandemics will be worse.

Figure 3) Melanin is widely spread in nature, and its main function is always to transform visible and invisible light into chemical energy by dissociating the water molecule, such as chlorophyll in plants. In the photograph, we can appreciate the seeds of an apple with a high melanin content, and the energy flow of melanin is the one that allows the hatching of the seeds.
Acknowledgements: This work was supported by an unrestricted grant of The Human Photosynthesis Study Center, S.C. Aguascalientes 2000, Mexico.
Disclosure of interest conflicts: none.
