Author(s): Ming C Liau*, Christine L Craig and Linda L Baker
The objective of this article is to rectify tumor shrinkage as a valid diagnosis of cancer therapy. Cancer incidence and cancer mortality keep on increasing, which are an indication that cancer has not been handled right. Perpetual proliferation of cancer cells (CCs) is the most outstanding feature of cancer. Elimination of CCs to shrink tumor became the commanding principle, which was wrong, because cancer evolved due to wound unhealing. Killing to create wound definitely is contra-indication of cancer therapy. The mistake was committed at a time when we did not have the full knowledge of cancer, which was excusable. Now we have better knowledge of cancer, but the mistake is carrying on to result in 10 million annual mortality worldwide in 2019 with an anticipated annual increment of 5%, which is not excusable. Cytotoxic therapies can only benefit a small minority of cancer patients in the early stage whose chem-surveillance have not yet fatally damaged, relying on the restoration of chemo-surveillance to subdue cancer stem cells (CSCs), which are resistant to cytotoxic agents. Under this circumstance, tumor shrinkage is a promising diagnosis toward remission. Cytotoxic therapies cause the death of a majority of cancer patients in the advanced stage whose chemo-surveillance have been fatally damaged. Under this circumstance, tumor shrinkage is an ominous diagnosis toward fatality. Tumor shrinkage should be used with discretion.
Cancer is a disease created by multiple factors. The collapse of chemo-surveillance or immuno-surveillance, the evolution of cancer stem cells (CSCs) from progenitor stem cells (PSCs) due to wound unhealing, and the progression of CSCs to faster replicating CCs by the activation of oncogenes or the inactivation of suppressor genes all contribute significantly to the development of cancer. An effective cancer therapy must be able to rectify all these contributing factors. Focusing on a specific factor is insufficient. The obsession to eliminate CCs to achieve tumor shrinkage is a grave mistake of cancer establishments to result in ever-increasing cancer mortality. Actually, elimination of CSCs is more important than the elimination of CCs, because CSCs contribute most fatal effects of cancer such as metastasis, drug resistance, anti-apoptosis, angiogenesis and recurrence. Induction of terminal differentiation by cell differentiation agent (CDA) formulations is the only option to eliminate CSCs which are critically linked to wound unhealing at the primary site. Induction of terminal differentiation can also result in the elimination of CCs, which are not as tightly linked to wound unhealing as CSCs. Terminal differentiation of CSCs and CCs cannot make the tumor to disappear. Thus, CDAs violated the commanding principle of tumor shrinkage put up by the cancer establishments, and were blocked as acceptable cancer drugs. CDA formulations are obviously the only drugs that can save the lives of advanced cancer patients whose chemo-surveillance have been fatally damaged. Oncologists and advanced cancer patients must unite to push for the approval of CDA formulations to save advanced cancer patients.
Perpetual proliferation of CCs is the most outstanding feature of cancer. Killing of proliferating cells naturally become the top choice of cancer therapy. Cytotoxic chemotherapy was actually a tragic byproduct of World War II. During the war, toxic mustard gas bombs were employed. Victims of toxic gas all displayed depletion of leukocytes in their blood specimens, which inspired oncologists to employ toxic chemicals to treat leukemia patients. Cytotoxic chemotherapy thus became a standard care of cancer, and the disappearance of CCs or tumor became a commanding principle for the judgment of the success of cancer therapy. Both were wrong! But the mistakes were made at a time when we did not have the full knowledge of cancer. Those mistakes were excusable. Cytotoxic chemotherapy and radiotherapy were the major cancer therapies employed during the war on cancer declared by President Nixon during 1971-1976, which was not successful to reduce cancer mortality [1]. Cancer establishments were aware that killing of CCs could not solve cancer, and started to search for other alternatives such as gene and targeted therapies during 1976-1996; anti-angiogenesis therapy during 1996-2016; and then to immunotherapy from 2016 onward presumably up-to 2036 [2]. Gene therapy was a right approach, because chromosomal abnormalities to activate oncogenes or to inactivate suppressor genes were a critical issue of cancer to speed up cell proliferation. But the attempt to develop gene therapy failed, because correction of chromosomal abnormalities was very difficult and expensive. Cancer establishments gave up, and turned to anti-angiogenesis approach, which was also a legitimate approach, because angiogenesis was an important feature of cancer to sustain the rapid growth of tumor mass. The successful attempt to terminate angiogenesis also results in internal bleeding to cause the death of cancer patients. Anti- angiogenesis was a failure as chemotherapy and radiotherapy that can kill CCs, but can also cause the death of cancer patients. Now, cancer establishments count on immunotherapy to win the war on cancer. Immunotherapy is an improved version of cytotoxic therapy to spare the adverse effects on normal stem cells. It is like targeted cancer therapy to focus on specific cancer targets, namely programed death target of cancer cells. But immunotherapy has the same problem of chemotherapy to show ineffectiveness against CSCs and to contribute to the damage of chemo-surveillance, which were the reasons behind the failure of chemotherapy and radiotherapy to win the war on cancer. So, immunotherapy is an improved version of cytotoxic therapy, but is not likely to turn around cancer mortality from increasing to decreasing. Elimination of CSCs through induction of terminal differentiation is the only option to save cancer patients [3-5]. CDA formulations are the best medicines to achieve elimination of CSCs to turn cancer mortality from increasing to decreasing [6-9].
To effectively solve cancer, we must understand how the problem of cancer evolves. Cancer is caused by multiple factors: the assaults to damage chemo-surveillance or immuno-surveillance, the evolution of CSCs from progenitor stem cells (PSCs) due to the collapse of chemo-surveillance, and the progression of CSCs to faster growing CCs through chromosomal abnormalities to activate oncogenes or to inactivate suppressor genes. All these factors play significant roles on the development of cancer. A perfect cancer therapy must be able to rectify all contributing factors of cancer [10].
Cancer evolves due to wound unhealing because of the collapse of chemo-surveillance. The concept of cancer evolves due to wound unhealing was first introduced by the great German pathologist Virchow in the 19th century [11]. It was again brought up by Dvorak in 1986 [12]. The close relationship between cancer and wound healing was noticed by MacCarthy-Morrough and Martin [13]. We provided the most important details on this subject that included abnormal methylation enzymes (MEs) to promote perpetual proliferation of CSCs and CCs by blocking differentiation [14-16]. chemo-surveillance as the nature’s creation of allosteric regulation on abnormal MEs for the perfection of wound healing to avoid disastrous consequences of wound unhealing, cancer being the worst consequence after differentiation inducers (DIs) and differentiation helper inducers (DHIs) as wound healing metabolites and active players of chemo-surveillance [17-19];. DIs are chemicals capable of eliminating telomerase from abnormal MEs and DHIs are inhibitors of MEs capable of potentiating the activity of DIs; hypomethylation of nucleic acids as a critical mechanism of terminal after differentiation mechanism of wound healing to involve the proliferation and the terminal differentiation of PSCs and the evolution of CSCs from PSCs due to wound unhealing through a single hit to silence ten-eleven translocator-1 enzyme [20-24]. These studies very convincingly establish that cancer evolves due to wound unhealing because of the collapse of chemo-surveillance. Our carcinogenesis studies also confirmed the validity of this concept. During the challenges with hepatocarcinogens, we noticed the appearance of numerous tiny hyperplastic nodules soon after the application of hepatocarcinogens which displayed abnormal MEs. These tiny hyperplastic nodules must represent the active wound healing by PSCs which express telomerase to turn MEs abnormal. Most of these tiny hyperplastic nodules disappeared soon afterward, which was an indication of the completion of wound healing, and only a few large size carcinomas appeared later from unhealed tiny hyperplastic nodules [25]. If Antineoplaston A10, which is phenylacetylglutamine, was provided during the challenges with hepatocarcinogens, hepatocarcinogenesis could be effectively prevented [26]. Phenylacetylglutamine is an effective anti-cachexia chemical. Cachexia symptoms are created by tumor necrosis factor (TNF), which is also named cachectin after its notorious effect to cause cachexia symptoms. A manifestation of cachexia symptoms is the excessive urinary excretion of low molecular weight metabolites due to hyperpermeability of blood vessels caused by TNF [27, 28]. Phenylacetylglutamine can effectively antagonize the effect of TNF to protect the functionality of chemo- surveillance to prevent carcinogenesis and to cure early stage cancer patients [17,26]. Chemo-surveillance is indeed an effective prescription of the nature to prevent and to cure cancer [17-19].
Wound healing is apparently an important health issue, so that the nature creates chemo-surveillance and immune-surveillance as protection mechanisms to ensure perfection of wound healing to avoid disastrous consequences of wound unhealing that can be tissue fibrosis, dementia, organ failure or cancer [29-32], chemo- surveillance to take care of wounds arising from toxic chemicals or physical means and immuno-surveillance to take care of wounds arising from infectious agents. So, chemo-surveillance and immuno-surveillance can act synergistically to eliminate wounds. Wound triggers biological and immunological responses [33]. Biological response involves the release of arachidonic acid (AA) by phospholipase A2 from membrane bound phosphatidylinositol for the synthesis of prostaglandins (PGs) by cyclooxygenases and PG synthases [33,34]. Although PGs are very active DIs, the induction of terminal differentiation of PSCs at the initial stage of wound is not the primary objective of PGs. Rather, the localized inflammation triggered by PGs is responsible for the increase of membrane permeability to facilitate the extravasation of plasma proteins and regulatory factors into the wound resulting in edema response that is the primary objective of PGs to orchestrate the healing process [35-37]. Chemo-surveillance mediated through DIs and DHIs normally functions as a brake to prevent the buildup of cells with abnormal MEs. This brake must be released for the cells with abnormal MEs to proliferate to heal the wound. PGs are metabolically unstable [34]. Their biological effects are most likely brief and confined to the wound area. Thus, the promotion of the proliferation of PSCs is the primary objective of PGs on wound healing, whereas the induction of the terminal differentiation of PSCs at the terminal stage of wound healing is accomplished by wound healing metabolites involved in chemo-surveillance. The stable end products of PGs, namely dicycloPGs which are not very active DIs, may then get involved in the induction of terminal differentiation of PSCs at the terminal stage of wound healing [36].
Biological response triggered by the wound is in general good for wound healing, but immunological response triggered by the wound is bad for wound healing. Immunological response prompts the patient to produce cytokines, which are often toxic proteins to cause damages to normal cells to produce therapeutic effects or pathological effects. TNF among cytokines is particularly bad for wound healing. On one hand it creates damages to produce wounds, and on the other hand it causes the collapse of chemo- surveillance to interfere wound healing. The outcome of wound healing is often determined by which effect is prevailing. If biological effect is prevailing, wound is quickly healed, and if immunological effect is prevailing, wound may not be healed to trgger clinical symptoms. The functionality of chemo-surveillance plays an important role to dictate the success or failure of wound healing [17-19]. Obviously, chemo-surveillance and immune- surveillance are the nature’s creations to act synergistically to heal wounds triggered by toxic chemicals or infectious agents. But immuno-surveillance can also act antagonistically to destroy chemo-surveillance by producing TNF. The interplays must be carefully examined to avoid antagonistic effects. In general, biological response always prevails in acute wounds to favor swift wound healing, and immunological response always prevails in persistent chronic wounds to result in wound unhealing.
Wound healing comes naturally without having to put up any effort, because the nature creates chemo-surveillance to ensure perfection of wound healing. Wound healing requires the proliferation and the terminal differentiation of PSCs. PSCs are the most primitive stem cells to initiate the development of organs or tissues during embryonic stage. A small fraction of these cells, usually less than 2% of the organ or tissue mass, are preserved in the organs or tissues for future expansion or repair. MEs of primitive embryonic stem cells including PSCs are abnormal like cancer cells due to association with telomerase [14-16]. Obviously, the seed of cancer is sawed at the very beginning of life, namely the fertilization of egg with sperm to activate the totipotent stem cell which expresses telomerase. The expression of telomerase among embryonic stem cells spreads through pluripotent stem cells, but secedes when pluripotent stem cells undergoing lineage transitions to reach unipotent stem cells. Therefore, abnormal MEs are a normal function of primitive stem cells during the embryonic stage. Disruption of the function of abnormal MEs during the embryonic stage of fetal development is detrimental as premature induction of terminal differentiation by thalidomide results in malformation of limbs. Abnormal MEs do not cause the problem of normal stem cells expressing telomerase because normal stem cells are protected by safety mechanisms such as contact inhibition, ten eleven translocator-1 (TET-1) enzyme to direct lineage transitions, and chemo-surveillance to prevent the build up of cells with abnormal MEs. If such safety mechanisms become dysfunctional, then the clinical symptoms arise [38- 40]. The collapse of chemo-surveillance forces PSCs to evolve into CSCs to escape contact inhibition. It takes a single hit to silence TET-1 enzyme to convert PSCs to become CSCs, which can be easily accomplished because PSCs are equipped with abnormally active MEs. But the proliferation of CSCs still cannot heal the wound, because the problem is the collapse of chemo- surveillance to accomplish terminal differentiation of PSCs, not the insufficiency of PSCs. The same mistake is repeated to force the progression of CSCs to become faster growing CCs by the translocations to activate oncogenes, or by the deletions to inactivate suppressor genes, eventually pushing CSCs to become full blown CCs. Thus, the valid interpretation of cancer evolution is the collapse of protection mechanisms of chemo-surveillance and immune-surveillance to result in wound unhealing that forces the evolution of PSCs to become CSCs and then to progress to faster growing CCs, all in an effort to heal the wound that cannot be healed in the first place. Healing the wound is, thus, the top priority of cancer therapy [18, 22, 23,41-44]. Creating wounds by cytotoxic agents as the commanding principle of cancer therapy adopted by the cancer establishments is definitely wrong. This commanding principle is a proven failure so far as the cancer mortality kept on increasing. Consequently, tumor shrinkage as a diagnostic criterion is also invalid for the assessment of the success of cancer therapy. President Biden of USA requested the health profession to reduce 50% cancer mortality in 25 years in 2022, which is a decrease of 2% mortality annually, that has not been accomplished as the cancer mortality is still on the way to increase at a rate of 0.2% annually in the USA, and 5% annually around the world according to the latest cancer statistics of ACS and NCI [54,46].
Whatever happens naturally is the nature’s creation to benefit living organisms. Photosynthesis is a prime example that produces oxygen free to sustain the lives of living organisms. Immuno-surveillance is another example to heal wounds arising from infectious agents, which is accepted by the health profession. Chemo-surveillance is a very important example to heal wound arising from toxic chemicals or physical means, which is not accepted by the health profession, because the cancer establishments are obsessed with toxic agents to kill CCs to combat cancer. Chemo-surveillance was a terminology we created to describe an observation that healthy people were able to maintain a steady level of metabolites active as DIs and DHIs, whereas cancer patients tended to show deficiency of such metabolites as shown in Table 1, which is reproduced from the reference [17].
|
Plasma/Urine Peptide Ratios |
CDA Levels |
Number of Patients |
% Distribution |
|
0.83-0.80 |
5.0 |
2 |
1.8 |
|
(Normal) |
|
|
|
|
0.80-0.60 |
4.3 |
7 |
6.5 |
|
0.60-0.40 |
3.1 |
18 |
16.7 |
|
(Responsive) |
|
|
|
|
0.40-0.20 |
1.9 |
38 |
35.2 |
|
0.20-0.10 |
0.9 |
24 |
22.2 |
|
0.10-0.02 |
0.37 |
19 |
17.6 |
|
(Unresponsive) |
|
|
|
Wound healing metabolites are hydrophobic metabolites that can be retained by adsorbants lsuch as C18 or XAD and recovered by organic solvents. Peptides share physical-chemical properties similar to wound healing metabolites. As a matter of fact, acidic peptides are very active DIs of Antineoplaston preparations purified from urine employing C18 as the adsorbant [17]. Therefore, peptides can be used as the surrogate molecules to represent wound healing metabolites for the analysis of the status of CDA levels of cancer patients. Peptides and hydrophobic metabolites were initially retained onto C18 cartridge from plasma deproteinized with sulfosalicylic acid, or urine without deproteinization treatment. After washing with water to remove unretained hydrophilic materials, the retained hydrophobic metabolites were recovered by 80% methanol. Solvent was removed by lyophilization, and the residue was dissolved in a small volume of water for HPLC resolution of peptide profile on a column of sulfonated polystyrene chromatographic system developed by Glenco Scitific Inc. of Houston, TX for peptide analysis. Results of 108 patients came to seek Antineoplaston therapy from Dr. Stanislaw R. Burzynski between 1982-1986 are presented in Table 1.
Peptide profiles of the plasma and urine are exactly the same, which are also very close to the peptide profile of spleen, but dissimilar to peptide profiles of other organs [47]. We believed that plasma peptides were primarily derived from the degradative products of erythrocytes, because spleen was the organ known to process dead erythrocytes. Uroerythrin was a major DHI of CDA-2 [48], which must be the degradative product of heme, also coming from erythrocytes. Acidic peptides, pigment peptides-0 (PP-0), which is membrane fragments containing phosphatidylinositol, and organic acid complexes (OA-0.79-0.83) which are diposomal complexes of AA or dicycloPGs with pregnenolone. The numerical number after PP or OA are the Kav values of chromatographic systems which may vary according to the particular chromatography employed. Acidic peptides are a major DI of Antineoplastons purified with C18 as the adsorbant, which are not present in CDA- 2 purified with XAD-16. PP-0 is a major active component DI of CDA-2, but is only a minor active component of Antineoplastons. Purification of anticancer chemicals from urine by C18 or XAD reverse phase chromatographies, may collect different active components, but both preparations exhibit comparable anticancer activities. Most active DHIs are well represented in the preparation of CDA-2 and Antineoplastons. In final analysis, both CDA-2 and Antineoplastons purified from urine are excellent cancer drugs to heal the wound that initiates the carcinogenesis process. It is our interpretation that healing wound is the major mechanism of anticancer effect of CDA-2 and Antineoplastons. But healing wound is not a major concern of cancer establishments. They preferred the opposite strategy to create wounds to kill CCs and to shrink tumor. They are still the bosses despite the failure to win the war on cancer declared by President Nixon in 1971, and to save advanced cancer patients. Cancer establishments have to step aside for cancer mortality to turnaround from increasing to decreasing.
Results presented in Table 1 clearly show that cancer patients in the early stage whose chemo-surveillance have not yet fatally damaged can benefit from cytotoxic therapies, marked as responsive above CDA level 3.1. Cancer progression tends to destroy chemo-surveillance like inflammatory diseases that create cachexia symptoms. Cytotoxic agents aggravate cachexia symptoms by creating more wounds. So, the collapse of chemo- surveillance is responsible for the evolution and the progression of cancer. The progression of cancer tends to lead to the collapse of chemo-surveillance. The application of cytotoxic agents further increases wounds to aggravate the collapse of chemo- surveillance. When the collapse has reached the critical level perhaps at CDA level of 3.1, patients are no longer responsive to further treatments. Cancer patients with CDA level above 3.1 can benefit from cytotoxic therapies, because after the elimination of CCs and slight damage to chemo-surveillance, chemo-surveillance may still be able to restore to subdue surviving CSCs, which are not responsive to cytotoxic agents protected by drug resistant and anti-apoptosis mechanisms [49-52]. The tumor shrinkage under such circumstance is a promising diagnosis toward remission. If CDA levels have been fatally destroyed below 3.1, there is no hope of the restoration of chemo-surveillance to subdue surviving CSCs, patients are either becoming unresponsive or even still responsive to reach complete remission will eventually succumb to recurrence. The tumor shrinkage under such circumstance is an ominous diagnosis toward fatality. Therefore, tumor shrinkage is not a valid diagnosis of the success of cancer therapy. The use of tumor shrinkage for the evaluation of cancer drugs is not a good idea and the use of tumor shrinkage to reject cancer drugs is a grave mistake of cancer establishments to result in the failure to solve cancer, because the solution of CSCs is essential to the success of cancer therapy, and CDA formulations are the only option to solve CSCs, which cannot make tumor to disappear [3-6,53]. The rule of tumor shrinkage cancer establishments put up is in essence blocks the success of cancer therapy. That is why cancer mortality keeps on increasing. We strongly recommended the use of CDA formulations to the rescue of metastasis, unresponsive and recurrent cancer patients to turn cancer mortality around from increasing to decreasing [9,54,55].
Cancer is basically a problem of growth regulation going awry. Abnormal MEs and chromosomal abnormalities are the most critical issues to account for the problems related to cancer. Abnormal MEs are responsible for the blockade of differentiation and abnormal chromosomal abnormalities are responsible for the speeding up of replication. Abnormal MEs start at the very beginning of the life, but chromosomal abnormalities happen quite late after the evolution of PSCs to become CSCs. Abnormal MEs are universal to all cancers, but chromosomal abnormalities are variable among different cancers. We are the only one to insist that abnormal MEs are the most critical issue of cancer, because abnormal MEs start at the very beginning of life and universal to all cancers [54,15]. Abnormal MEs play an essential role for the development of fetus and wound healing without causing problems, because there are protection mechanisms to restrict the operation of abnormal MEs. When such protection mechanisms break down, abnormal MEs become serious clinical problems, often fatal as above described. Chromosomal abnormalities happen late after the establishment of CSCs and variable among different cancers. The solution of chromosomal abnormalities is very difficult and expensive. In fact, cancer establishments invested 20 years between 1976-1996 to develop gene therapy, only to learn the difficulty of gene therapy and to give up. Gene therapy is fascinating, but is not feasible. It is very difficult to solve gene abnormalities such as translocation or deletion. Even a difficult gene abnormality is solved. There may soon popup another gene abnormality to negate the previous effort. Induction of terminal differentiation can provide an easier solution of chromosomal abnormalities. Afterall, oncogenes and suppressor genes are cell cycle regulatory genes. They have important roles to play when cells are in cell cycle replicating. But if replicating cells are forced to exit cell cycle to undergo terminal differentiation, they have no roles to play. So, induction of terminal differentiation by CDA formulations can also put to rest the problems of chromosomal abnormalities. Of course, killing of CCs can also put to rest chromosomal abnormalities. That has been tested as a presidential project during 1971-1976 and thereafter up to now, but failed. Cytotoxic cancer therapy is not a good solution of cancer!.
MEs are ternary enzyme complex consisting of methionine adenosyltransferase (MAT)-methyl transferase (MT)-S- adenosylhomocysteine hydrolase (SAHH) which play a pivotal role on the regulation of cell replication and differentiation [55]. Because of this pivotal regulatory role, MEs are subjected to exceptional allosteric regulation [56]. Allosteric regulation is the most pervasive biological regulation [57]. MEs are exceptionally subjected to double allosteric regulations: on the individual enzymes by steroid hormone, and on the enzyme complex by telomerase and chemo-surveillance [5,16]. Steroid hormone promotes the formation of stable and active ternary MEs. The association of ternary MEs with telomerase further increases the stability and the activity of MEs [58]. The association of ternary MEs with telomerase changes the kinetic properties of MAT- SAHH isozyme pair and the regulation greatly in favor of cell growth. Telomerase associated MAT-SAHH isozyme pair display Km values 7-fold higher than the normal isozyme pair [14-16]. The higher Km values suggest that cells expressing telomerase have a larger pool sizes of S-adenosylmethionine (AdoMet) and S-adenosylhomocysteine (AdoHcy), which are important for the promotion of the growth of cells expressing telomerase as the study of Prudova et al. [59] indicated that protein associated with AdoMet could increase the stability against protease digestion, and the study of Chiva et al. [60] indicated that when cancer cells were induced to undergo terminal differentiation, the pool sizes of AdoMet and AdoHcy shrank greatly. Obviously, abnormal MEs play an important role on cell growth. They are the bullseye of cancer target [61]. When this target is hit, the other problems promoting cancer such as gene abnormalities and chemo-surveillance will also fall. Destabilization of abnormal MEs offers a perfect cancer therapy [6,24].
CDA Formulations as the Only Option for the Solution of CSCs CSCs like their precursors PSCs constitute only a small minority of the mass, usually less than 2%. CSCs of malignant brain tumors are exceptional to have more than 10% of CSCs in the primary tumor mass [62,63]. When a tumor has CSCs more than 10% of the mass like primary brain tumors, it become unresponsive to cytotoxic therapies, because these cells are protected by drug resistant and anti-apoptosis mechanisms [49-52]. Cytotoxic cancer therapies tend to drive up the content of CSCs [64]. Cancer patients receiving cytotoxic therapies often become unresponsive after a prolong treatment. Finding effective treatments to eliminate CSCs is essential to the success of cancer therapy [5]. Myelodysplastic syndromes (MDSs) are a unique case to search for drugs effective against CSCs.
MDSs often start with a display of immunological disorder, which prompts the local production of inflammatory cytokines [65]. Among such cytokines, TNF is the critical factor related to the development of MDSs [66]. It causes excessive apoptosis of bone marrow stem cells, thus, severely affecting the ability of the patient to produce hematopoietic cells such as erythrocytes, platelets or neutrophils. TNF is also responsible to trigger cachexia symptoms resulting in the collapse of chemo-surveillance as above described. As a consequence, chemo-surveillance normally operating in healthy people to keep cells with abnormal MEs in check becomes dysfunctional to force the evolution of CSCs from PSCs, and then the progression of CSCs to full blown CCs. The propagating pathological cells of MDSs have been identified as human CSCs [67]. Therefore, MDSs are diseases of cancer development at the stage of CSCs.
Vidaza, Decitabine and CDA-2 are the three drugs approved by the Chinese FDA for the therapy of MDSs. Vidaza and Decitabine are also the two drugs approved by the US FDA for the therapy of MDSs. Professor Jun Ma, Director of Harbin Institute of Hematology and Oncology, was instrumental in conducting clinical trials of all three MDSs drugs. According to his assessments based on two cycles of treatment protocols each 14 days, CDA-2, which was our invention of the preparation of wound healing metabolites from urine, had a noticeable better therapeutic efficacy based on the cytological evaluation, although slower to reach complete remission, and a markedly better therapeutical efficacy based on the hematological improvement evaluation, namely becoming independent on blood transfusion to stay alive, as shown in Figure 1, which is reproduced from the reference [69].
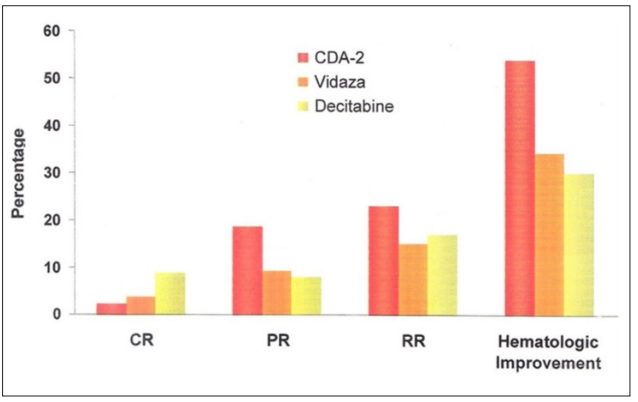
Figure 1: Relative Effectiveness of MDSs Drugs
Therapy of MDSs require the conversion of pathological CSCs to become functional erythrocytes, platelets or neutrophils. Killing of CSCs cannot cure MDSs. Therefore, induction of terminal differentiation of CSCs is the only option for the therapy of MDSs. CDA-2 employs wound healing metabolites to destabilize abnormal MEs and phenylacetylglutamine to antagonize TNF to restore chemo-surveillance to accomplish the therapy of MDSs as above described, whereas Vidaza and Decitabine rely on the covalent bond formation between MT and 5-aza-cytosine incorporated into DNA to inactivate MEs [70]. The action of CDA-2 is selective on the tumor factor of telomerase, whereas the action of Vidaza and Decitabine is non-selective that can also affect normal stem cells. Thus, CDA-2 is devoid of adverse effects, whereas Vidaza and Decitsabine are proven carcinogens, and very toxic to DNA [71-75].
Clearly, CDA-2 is the drug of choice for the therapy of MDSs with better therapeutic efficacy and devoid of adverse effects. It should be considered the standard care of CSCs as the solution of CSCs of the primary site is critically linked to wound unhealing [3-9].
Solution of CSCs is very critical to the success of cancer therapy [3-9]. Of course, cancer establishments were aware of the importance of CSCs on cancer therapy. The pharmaceutical giant GSK put up 1.4 billion, the most expansive investment on cancer drugs, to develop monoclonal antibodies against CSCs invented by the scientists of Stanford University about 17 years ago, which did not materialized, because killing of CSCs was not an option to solve the issue of CSCs. The cancer establishments were trapped in the killing of CSCs and CCs to solve cancer that did not work. They are the bosses. They can exercise their power to block CDA formulations that can solve the issue of CSCs. In final analysis, cancer can be easily solved by pursuing would healing process [3-10,24,38,42-45,54,55,68,69]. It is not solved, because the cancer establishments are pursuing the opposite to kill CCs to create wounds.
Cancer is a big health issue. It is the top killer or the second top killer around most countries. Solution of cancer is a national interest to almost every country. That was the reason President Nixon declared war on cancer in 1971. We did not have enough knowledge on cancer at that time to win the war on cancer. But now we have complete knowledge on cancer to win the war on cancer. We have learned enough from the failures to make the correction to put cancer away. Inability to solve the issue of CSCs is the biggest factor to contribute to the failure of cancer therapy in the past. Surgery is apparently the top choice of cancer therapy when CSCs and CCs are confined to the primary site. A surgical removal of the primary tumor instantly solves the problem of cancer. Healing of surgical wounds comes naturally in a week or two. Metastasis limits the use of surgery as a treatment modality, since surgical wounds tend to disseminate metastasis. Metastasis is the making of CSCs [76]. If CSCs can be effectively put under control. Even cancer patients showing evidence of metastasis are still eligible candidates for the surgery [55]. CDA formulations are the best drugs to take care of CSCs as above described. A combination of surgery and CDA formulations can win the war on cancer. Likewise, ineffectiveness against CSCs and the contribution to the damage to chemo-surveillance account for the failure of cytotoxic therapies in the past, which can be remedied with CDA formulations. So, a combination of CDA formulations and cytotoxic therapies, including immunotherapy can help to save a lot of cancer patients [8,9, 42,44,54]. CDA formulations are very critical to the success of cancer therapy. Surgeons, oncologists and cancer patients must unite to push for the approval of CDA formulations for the perfection of cancer therapy to win the war on cancer. We have carried out extensive studies on natural and non- natural DIs and DHIs for the manufacture of CDA formulations [2,3,7,9,10,35, 36,38,48,68,77-81].
Cancer is caused by multiple factors that include the collapse of chemo-surveillance, the evolution of CSCs from PSCs due to the collapse of chemo-surveillance to cause wound unhealing, and the progression of chromosomal abnormalities to activate oncogenes or to inactivate suppressor genes. Cancer therapy had a bad start to rely on toxic chemicals to kill CCs to reduce tumor size. Tumor shrinkage became a commanding principle, which was a promising diagnosis toward remission when chemo-surveillance was not fatally damaged. But tumor shrinkage became an ominous diagnosis when chemo-surveillance was fatally damaged. Tumor shrinkage is, therefore, not a valid diagnosis of the success of cancer therapy. The success of cancer therapy depends on the elimination of CSCs. The induction of terminal differentiation of CSCs is the only option to solve the issue of CSCs. The solution of CSCs is essential to the success of cancer therapy. Surgeons, oncologists and cancer patients must unite to push for the approval of CDA formulations for the perfection of cancer therapy to win the war on cancer.
We are grateful for the support on the studies of abnormal methylation enzymes by Professor Robert B. Hurlbert of University of Texas MD Anderson Cancer Center and Professor George CY Chiou of Texas A&M University Medical Center, the support of the studies of wound healing metabolites and chemo-surveillance by Dr. Stanislaw R. Burzynski of Burzynski Research Institute of Stafford, TX, the support of clinical development of CDA-2 by Mr. Ringo ML Chang of Everlife Pharmaceutical Co. of Hefei, Anhui, China, the development of CDA formulations by Professor John P. Fruehauf of Choa’s Family Cancer Comprehensive Center of University of California Irvine Medical Center. We appreciate very much the encouragement from President Joe Biden on the development of CDA formulations through personal communications.
The authors declare. no conflicts of interest.
