Author(s): Pourya Zarshenas
The drug delivery application of Porous metal–organic frameworks (MOFs) have been investigated due to their unique structures which are built of inorganic nodes and organic ligands. In present study, Uio-67-5-FU was successfully prepared by applied for delivery of 5-fluorouracil (5-FU). Using variety of analytical methods including FTIR, FESEM, EDS, and the prepared nanostructure was characterized. Results revealed the placement of the drug in zeolite is well done and also the in vitro loading and releasing studies, for 5-FU was evaluated. In addition, Based on the in vitro cytotoxicity results, Uio-67-5Fu was able to increase cytotoxicity compared to that of 5-Fu on HT-29 cancerous cells indicating the highlighted role of this drug delivery system.

Figure 1: Placement of drug in the structure of MOF
Cancer as the most prevalent diseases worldwide is one of the main public health concerns. In spite of intensive efforts for treatment of cancer, the necessity of developing effective agents isn’t ignorable [1]. Designing an ideal drug delivery system for targeting cancer cell is considered as a hot topic in life science research. MOFs with crucial features including high drug loading capacity, high surface area, as well as tunable pore size is used for drug delivery intensively [2]. MOFs plays an important role as an carriers in drug delivery because they are non-toxic as well as the uptake of drugs and getting across the cell membrane has been facilitated via controlling the size of MOFs [3].
5-florouracil (5-FU) is anticancer drugs which is able to induces cytotoxic and increase DNA damage [4]. Although, 5 FU frequently applied, developed drug resistance and severe side effects affected its clinical application [5]. Encapsulate of 5-FU using various DDS could be an effective idea [6]. In present work, the drug loading capacity of i for 5-FU as an anticancer drug was evaluated. Upon exposure by Uio-67-5Fu the in vitro cytotoxicity against cancer cells were assessed.
Finally but contrary to the original goal of this project, which was to use a muff, because of the simpler and faster synthesis, we carried out this project with a MOF.
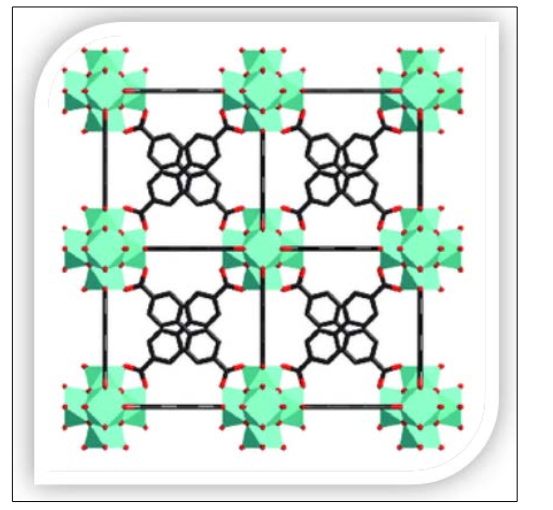
Figure 2: Placement of Structures of UiO-67[26]
UiO-67 is a Zirconium-based metal-organic framework that features larger pore sizes compared to UiO-66. This is due to the longer structure of BPDC (UiO-67) as compared to BDC (UiO-66).
The structure and properties of two new UiO-67-type metal- organic frameworks, along with their linker synthesis and powder and single crystal synthesis, are presented. The new MOFs, UiO67-Me and UiO-67-BN, are based on 3,3?-dimethylbiphenyl and 1,1-binaphthyl linker scaffolds, and show a much higher stability to water than the thoroughly investigated UiO-67, which is based on the biphenyl scaffold. On the basis of structure models obtained from single crystal X-ray diffraction, it is seen that these linkers are partly shielding the Zr cluster. The new materials have higher density than UiO-67, but show a higher volumetric adsorption capacity for methane. UiO-67-BN exhibits excellent reversible water sorption properties, and enhanced stability to aqueous solutions over a wide pH range; it is to the best of our knowledge the most stable Zr-MOF that is isostructural to UiO-67 in aqueous solutions.
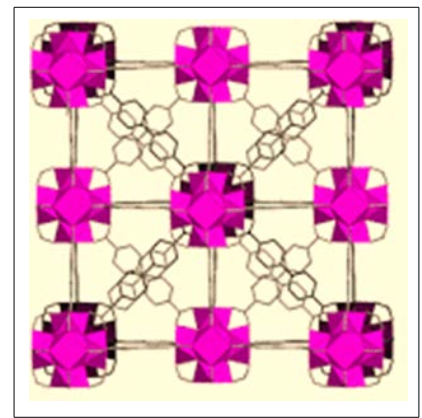
Figure 3: Placement of Structures of UiO-67
• Gas adsorption and storage:
o Carbon dioxide (CO2)
o Hydrogen (H2)
o Methane (CH4)
• Neutralisation/detoxication of chemical warfare agents (nerve
gas)
• Catalyst
• Biomolecular motor
• Biomedical imaging
Properties
Formula: Zr6O4(OH)4(BPDC)6
Surface area: up to 1970+ m2/gr (BET)
he phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material [7].
The idea behind Nanocomposite is to use building blocks with dimensions in nanometre range to design and create new materials with unprecedented flexibility and improvement in their physical properties. In the broadest sense this definition can include porous media, colloids, gels and copolymers, but is more usually taken to mean the solid combination of a bulk matrix and nano-dimensional phase(s) differing in properties due to dissimilarities in structure and chemistry [8].
The mechanical, electrical, thermal, optical, electrochemical, catalytic properties of the nanocomposite will differ markedly from that of the component materials. Size limits for these effects have been proposed.
1. 5 nm for catalytic activity 2. <20 nm for making a hard magnetic material soft 3. <50 nm for refractive index changes 4. <100 nm for achieving superparamagnetism, mechanical strengthening or restricting matrix dislocation movement [9].
Nanocomposites are found in nature, for example in the structure of the abalone shell and bone [10]. The use of nanoparticle-rich materials long predates the understanding of the physical and chemical nature of these materials. Some researchers investigated the origin of the depth of color and the resistance to acids and bio-corrosion of Maya blue paint, attributing it to a nanoparticle mechanism. From the mid-1950s nanoscale organo-clays have been used to control flow of polymer solutions (e.g. as paint viscosifiers) or the constitution of gels (e.g. as a thickening substance in cosmetics, keeping the preparations in homogeneous form). By the 1970s polymer/clay composites were the topic of textbooks, although the term “nanocomposites” was not in common use [11]. In mechanical terms, nanocomposites differ from conventional composite materials due to the exceptionally high surface to volume ratio of the reinforcing phase and/or its exceptionally high aspect ratio. The reinforcing material can be made up of particles (e.g. minerals), sheets (e.g. exfoliated clay stacks) or fibers (e.g. carbon nanotubes or electrospun fibers) [12]. The area of the interface between the matrix and reinforcement phase(s) is typically an order of magnitude greater than for conventional composite materials [13]. The matrix material properties are significantly affected in the vicinity of the reinforcement. Some scientists be aware that with polymer nanocomposites, properties related to local chemistry, degree of thermoset cure, polymer chain mobility, polymer chain conformation, degree of polymer chain ordering or crystallinity can all vary significantly and continuously from the interface with the reinforcement into the bulk of the matrix. This massive quantity of reinforcement surface area means that a relatively small amount of nanoscale reinforcement can have an observable effect on the macroscale properties of the composite [14].
Metal-organic frameworks (MOFs) are a class of compounds consisting of metal ions or clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. They are a subclass of coordination polymers, with the special feature that they are often porous. The organic ligands included are sometimes referred to as “struts” or “linkers”, one example being 1,4-benzenedicarboxylic acid (BDC).
More formally, a metal-organic framework is a coordination network with organic ligands containing potential voids. A coordination network is a coordination compound extending, through repeating coordination entities, in one dimension, but with cross-links between two or more individual chains, loops, or spiro-links, or a coordination compound extending through repeating coordination entities in two or three dimensions; and finally a coordination polymer is a coordination compound with repeating coordination entities extending in one, two, or three dimensions [1].
In some cases, the pores are stable during elimination of the guest molecules (often solvents) and could be refilled with other compounds. Because of this property, MOFs are of interest for the storage of gases such as hydrogen and carbon dioxide. Other possible applications of MOFs are in gas purification, in gas separation, in water remediation, in catalysis, as conducting solids and as supercapacitors [2,3].
The synthesis and properties of MOFs constitute the primary focus of the discipline called reticular chemistry (from Latin reticulum, “small net”) [4]. In contrast to MOFs, covalent organic framework (COFs) are made entirely from light elements (H, B, C, N, and O) with extended structures.
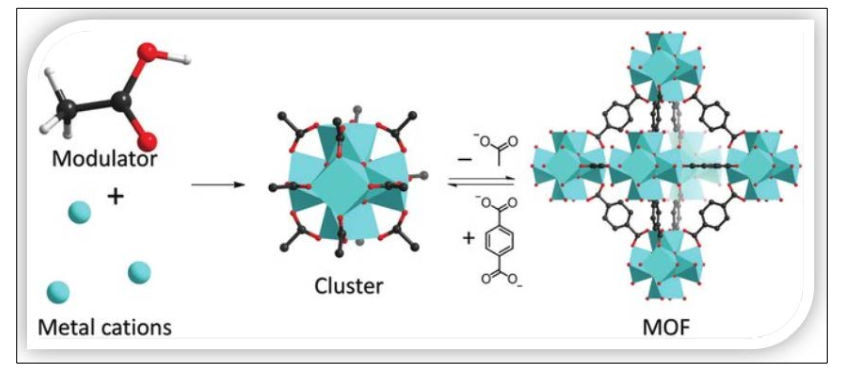
Figure 4: Representative modulated synthesis [26]
MOFs are composed of two major components: a metal ion or cluster of metal ions and an organic molecule called a linker. For this reason, the materials are often referred to as hybrid organic-inorganic materials; however, this terminology has recently been explicitly discouraged [1]. The organic units are typically mono-, di-, tri-, or tetravalent ligands [6]. The choice of metal and linker dictates the structure and hence properties of the MOF. For example, the metal’s coordination preference influences the size and shape of pores by dictating how many ligands can bind to the metal and in which orientation.
To describe and organize the structures of MOFs, a system of nomenclature has been developed. Subunits of a MOF, called secondary building units (SBU), can be described by topologies common to several structures. Each topology, also called a net, is assigned a symbol, consisting of three lower-case letters in bold. MOF-5, for example, has a pcu net.
Attached to the SBUs are bridging ligands. For MOF’s, typical bridging ligands are di- and tricarboxylic acids. These ligands typically have rigid backbones. Examples are benzene-1,4- dicarboxylic acid (BDC or terephthalic acid, biphenyl-4,4’- dicarboxylic acid (BPDC), and the tricarboxylic acid trimesic acid.
The study of MOFs developed from coordination chemistry and solid state inorganic chemistry, especially the zeolites.
Except for the use of preformed ligands, MOFs and zeolites are produced almost exclusively by hydrothermal or solvothermal techniques, where crystals are slowly grown from a hot solution. In contrast with zeolites, MOFs are constructed from bridging organic ligands that remain intact throughout the synthesis [8]. Zeolite synthesis often makes use of a “template”. Templates are ions that influence the structure of the growing inorganic framework. Typical templating ions are quaternary ammonium cations, which are removed later. In MOFs, the framework is templated by the SBU (secondary building unit) and the organic ligands. A templating approach that is useful for MOFs intended for gas storage is the use of metal-binding solvents such as N,Ndiethylformamide and water. In these cases, metal sites are exposed when the solvent is evacuated, allowing hydrogen to bind at these sites. Four developments were particularly important in advancing the chemistry of MOFs.
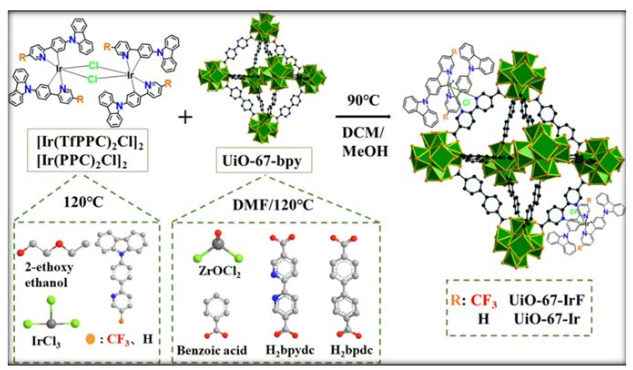
Figure 5: Synthesis routine for UiO-67-IrF [28]
Since ligands in MOFs typically bind reversibly, the slow growth of crystals often allows defects to be redissolved, resulting in a material with millimeter-scale crystals and a near-equilibrium defect density. Solvothermal synthesis is useful for growing crystals suitable to structure determination, because crystals grow over the course of hours to days. However, the use of MOFs as storage materials for consumer products demands an immense scale-up of their synthesis. Scale-up of MOFs has not been widely studied, though several groups have demonstrated that microwaves can be used to nucleate MOF crystals rapidly from solution. This technique, termed “microwave-assisted solvothermal synthesis”, is widely used in the zeolite literature, and produces micron-scale crystals in a matter of seconds to minutes, in yields similar to the slow growth methods. Some MOFs, such as the mesoporous MIL-100(Fe), can be obtained under mild conditions at room temperature and in green solvents (water, ethanol) through scalable synthesis methods.
A solvent-free synthesis of a range of crystalline MOFs has been described. Usually the metal acetate and the organic proligand are mixed and ground up with a ball mill. Cu3 (BTC)2 can be quickly synthesised in this way in quantitative yield. In the case of Cu3 (BTC)2 the morphology of the solvent free synthesised product was the same as the industrially made Basolite C300. It is thought that localised melting of the components due to the high collision energy in the ball mill may assist the reaction. The formation of acetic acid as a by-product in the reactions in the ball mill may also help in the reaction having a solvent effect in the ball mill. It has been shown that the addition of small quantities of ethanol for the mechanochemical synthesis of Cu3 (BTC)2 significantly reduces the amounts of structural defects in the obtained material.
A recent advancement in the solvent-free preparation of MOF films and composites is their synthesis by chemical vapor deposition. This process, MOF-CVD, was first demonstrated for ZIF-8 and consist of two steps [19]. In a first step, metal oxide precursor layers are deposited. In the second step, these precursor layers are exposed to sublimed ligand molecules that induce a phase transformation to the MOF crystal lattice. Formation of water during this reaction plays a crucial role in directing the transformation. This process was successfully scaled up to an integrated cleanroom process, conforming to industrial microfabrication standards [20].
Numerous methods have been reported for the growth of MOFs as oriented thin films. However, these methods are suitable only for the synthesis of a small number of MOF topologies. One such example being the vapor-assisted conversion (VAC) which can be used for the thin film synthesis of several UiO-type MOFs [21].
High-Throughput (HT) methods are a part of combinatorial chemistry and a tool for increasing efficiency. Basically, there are two synthetic strategies within the HT-methods: On the one hand the combinatorial approach, here all reactions take place in one vessel, which leads to product mixtures and on the other hand the parallel synthesis, here the reactions take place in different vessels. Furthermore, a distinction is made between thin films and solvent-based methods [22].
Solvothermal synthesis can be carried out conventionally in a teflon reactor in a convection oven or in glass reactors in a microwave oven (high-throughput microwave synthesis). The use of a microwave oven changes, in part dramatically, the reaction parameters. In addition to solvothermal synthesis, there have been advances in using supercritical fluid as a solvent in a continuous flow reactor. Supercritical water was first used in 2012 to synthesize copper and nickel-based MOFs in just seconds [23]. In 2020, supercritical carbon dioxide was used in a continuous flow reactor along the same time scale as the supercritical water-based method, but the lower critical point of carbon dioxide allowed for the synthesis of the zirconium-based MOF UiO-66 [24].
In high-throughput solvothermal synthesis, a solvothermal reactor with (eg) 24 cavities for Teflon reactors is used. Such a reactor is sometimes referred to as a multiclav. The reactor block or reactor insert is made of stainless steel and contains 24 reaction chambers, which are arranged in four rows. With the miniaturized Teflon reactors, volumes of up to 2 mL can be used. The reactor block is sealed in a stainless steel autoclave; for this purpose, the filled reactors are inserted into the bottom of the reactor, the Teflon reactors are sealed with two Teflon films and the reactor top side is put on. The autoclave is then closed in a hydraulic press. The sealed solvothermal reactor can then be subjected to a temperature-time program. The reusable Teflon film serves to withstand the mechanical stress, while the disposable Teflon film seals the reaction vessels. After the reaction, the products can be isolated and washed in parallel in a vacuum filter device. On the filter paper, the products are then present separately in a socalled sample library and can subsequently be characterized by automated X-ray powder diffraction. The informations obtained are then used to plan further syntheses.
During drying it comes to the removal of free and bound water from the crystal grid, which is then counterbalanced back in contact with materials such as stored grain and feed, pet litter, in flue gas to prevent condensation and the like [19]. Clinoptilolite stabilize moisture at a low dose of volume and avoid the adverse effects of water [20].
Metal-organic frameworks (MOFs) are organic-inorganic hybrid crystalline porous materials that consist of a regular array of positively charged metal ions surrounded by organic ‘linker’ molecules. The metal ions form nodes that bind the arms of the linkers together to form a repeating, cage-like structure. Due to this hollow structure, MOFs have an extraordinarily large internal surface area.
Researchers have synthesized MOFs that feature a surface area of more than 7800 square meters per gram. To put this into context, if you could lay out the available surface area in a teaspoon of this material (around a gram of solid), it would cover an entire soccer field. MOFs offer unique structural diversity in contrast to other porous materials uniform pore structures; atomic-level structural uniformity; tunable porosity; extensive varieties; and flexibility in network topology, geometry, dimension, and chemical functionality. This allows researchers the successful control of framework topology, porosity, and functionality.
MOFs unique structure design and tunability - crystalline porous materials that are composed of both organic and inorganic components in a rigid periodic networked structure- is not readily accessible in conventional porous materials, e.g., purely inorganic zeolites. By making the MOF from different metal atoms and organic linkers, researchers can create materials that selectively absorb specific gases into tailor-made pockets within the structure. MOFs therefore offer great potential for their effective integration and exploration in various sensing applications. MOFs can be put together arbitrarily like Lego bricks and outperform every previously known class of material in terms of flexibility.
The physicochemical properties of materials are governed by the synergistic effects of structures and compositions, and MOFs are fascinating examples of how the unique structure of hollowstructured materials can provide a whole raft of advantageous features. Among them are enhanced surface-to-volume ratio; low density; microreactor environment; higher loading capacities; and reduced transmission lengths of mass and charge.
Consequently, the preparation of hollow structures for technological applications has long been a popular research field for chemists and materials scientists. However, the synthesis of porous or hollow-structured materials with controllable - and especially complex - structures and certain composition in a controlled manner has always been a challenge for scientists.
Consequently, the preparation of hollow structures for technological applications has long been a popular research field for chemists and materials scientists. However, the synthesis of porous or hollow-structured materials with controllable - and especially complex - structures and certain composition in a controlled manner has always been a challenge for scientists.
Numerous applications in many fields are being developed that exploit MOFs’ cage-like structure, such as gas storage and separation, liquid separation and purification, electrochemical energy storage, catalysis, and sensing. In addition to direct applications, MOFs have been used as unique precursors for the construction of inorganic functional materials with unparalleled design possibilities, such as carbons, metal-based compounds, and their composites. Currently, carbonaceous materials are attracting much interest for their extensive applications including adsorption, catalysis, batteries, fuel cells, supercapacitors, and drug delivery and imaging. In addition, some sensors are also one of the important applications of carbonaceous materials, because they are closely related to human health.
There are varieties of approaches for the preparation of these carbon materials, but among them, directly carbonizing from organic precursors is the most frequently used method to prepare nanoporous carbons due to its flexibility and simplicity. These materials present certain drawbacks, though, such as low surface areas, disordered structures, and non-uniform sizes, which will greatly limit their applications. However, researchers found that carbon materials derived from metal-organic frameworks (MOFs) could overcome these limitations.
Fluorouracil is also known as FU or 5FU and is one of the most
commonly used drugs to treat cancer. It is most often used in
combination with other cancer drugs to treat many types of cancer
including:
• breast cancer
• head and neck cancers
• anal cancer
• stomach cancer
• colon cancer
• some skin cancers (as a cream)
Fluorouracil (5-FU), sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer. By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer. As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts.
Side effects of use by injection are common. They may include inflammation of the mouth, loss of appetite, low blood cell counts, hair loss, and inflammation of the skin. When used as a cream, irritation at the site of application usually occurs. Use of either form in pregnancy may harm the baby. Fluorouracil is in the antimetabolite and pyrimidine analog families of medications. How it works is not entirely clear but believed to involve blocking the action of thymidylate synthase and thus stopping the production of DNA. Fluorouracil was patented in 1956 and came into medical use in 1962. It is on the World Health Organization’s List of Essential Medicines.
Fluorouracil is part of a group of chemotherapy drugs known as anti-metabolites. Anti-metabolites are similar to normal body molecules but they have a slightly different structure. These differences mean that anti metabolites stop cancer cells working properly. They stop the cells making and repairing DNA. Cancer cells need to make and repair DNA so that they can grow and multiply.
You have fluorouracil into your bloodstream. You may have fluorouracil through a drip or using a small pump that you carry around for several days.
Fluorouracil is also available as an ointment for skin cancer. When used as an ointment it does not cause the usual side effects but it can cause temporary irritation and inflammation in the treated areas of skin into your bloodstream.
You might have treatment through a long plastic tube that goes
into a large vein in your chest. The tube stays in place throughout
the course of treatment. This can be a:
• central line
• PICC line
• Portacath
You usually have fluorouracil as part of a course of several cycles of treatment. You generally have up to 6 cycles of treatment. Each cycle lasts 2, 3 or 4 weeks. You have continuous treatment through a small portable pump. The nurse attaches it to your central line. This means you can go home with it. You go back to hospital regularly for the nurse to change your pump, and to see how you’re doing.
You have blood tests before and during your treatment. They check your levels of blood cells and other substances in the blood. They also check how well your liver and kidneys are working
How often and how severe the side effects are can vary from person to person. They also depend on what other treatments you’re having. For example, your side effects could be worse if you’re also having other drugs or radiotherapy
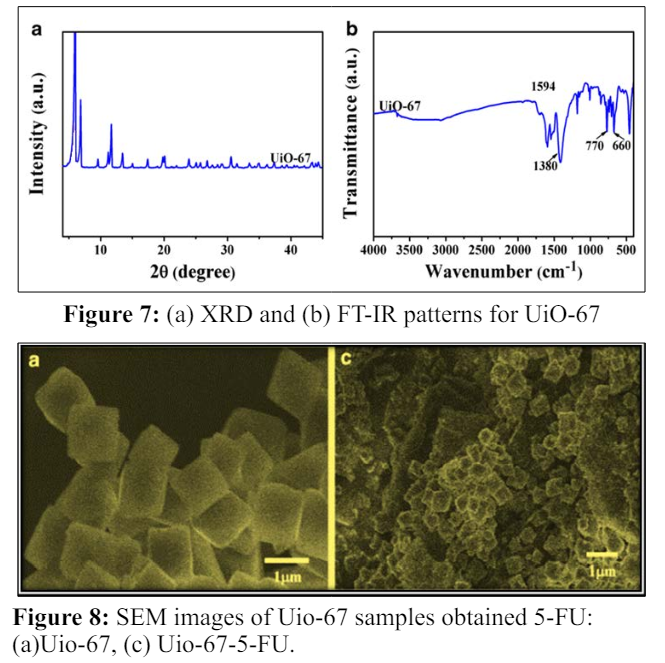
The chemical structure of the Uio-67-5Fu was characterized with different analytical methods such as XRD, SEM & TEM.
Figure 6: TEM image of Uio-67-5Fu
The MOF- with the proper size and the accessible porosity could be used for loading and release of 5-Fu. The loading capacity of UiO-67 under physiological condition (pH 7.4) was investigated. The results showed high drug loading capacity (DLC) (90%) and drug loading efficiency (DLE) 70% by UV-Vis spectroscopy. The results of release profiles of UiO-67-5Fu revealed sustained for 72 h despite with an initial rapid release.
In order to determine the in vitro cytotoxicity of the UiO-67, 5-FU drug, and UiO-67-5Fu HT-29 cell lines, MTT assay was conducted. The obtained results of the cell viability assay showed that UiO-67-5Fu and 5-FU drug inhibited cell growth in a time and dose-dependent manner while the UiO-67- showed less growth inhibition after 48 h compared to drug loaded UiO-67 and free drug 5-FU. Based on this results, one may conclude that MOFs with low toxicity could be used effectively for biological applications in the future [3].
In this study, UiO-67 was applied for delivery of 5-Fu. The obtained nanostructure poses spherical morphology with an average diameter of 39-52 nm. Results showed the high loading capacity (90%) and sustained drug release behavior. Moreover, upon exposure by UiO-67-5Fu, higher cytotoxicity than those for UiO-67 and 5-Fu drug against PC3 cells was determined indicating UiO-67-5Fu may could be a promising anticancer drug delivery system in the future.
The numerous advantages of MOFs, foremost their high surface area and modular composition, place them at a multidisciplinary crossroads. For good reason, MOFs are one of the most active research fields today, with aspects of their fundamental and applied properties permeating into disciplines as varied as electronics, chemical engineering, and optics. Whereas this Outlook does not attempt to delineate the developments and potential in all these areas, we have introduced some of the exciting prospects related to continued synthetic advances in the field. We further elaborated on three applied areas where MOFs are primed to excel: in challenging gas separations, as porous electrical conductors, and in heterogeneous catalysis. These examples are not exhaustive, but present subtleties that are applicable and relevant to many other applications of MOFs. The challenges and opportunities in these select applications, which span both the traditional and the modern aspects of the field, are illustrative of the continually expanding interest and bright future for MOF chemistry.
