Author(s): Amelia Morgillo*, Caterina Rosaria Morgillo, Angela Cambareri and Edoardo Marovino
Introduction: The use of benzodiazepines (BDZ) is notoriously associated with significant long-term problems and it is estimated that the long-term users (LTU) in Italy are over 3 million people, including many elderly people. Unfortunately, 40 to 80% of the LTUs develop dependence and many also have tolerance, with the need for a progressive increase in the daily dosage up to the point of tolerating daily megadoses. In recent years, the off-label use of continuous infusion subcutaneous flumazenil has established itself as a viable approach for rapid hospital detoxification of these sometimes very complex clinical cases. The purpose of the article is, starting from the pharmacological and biochemical bases, to describe the mechanism of action, the areas of applicability and the possible criticalities by analyzing the Italian data.
Materials and Methods: A computerized research was carried out for the articles to be inserted through use of international databases PUBMED and RESEARCHGATE by typing in keywords such as “flumazenil, high dose benzodiazepine users, use of flumazenil for benzo detoxification” and related articles. We also used the PUBCHEM database to describe some chemical and pharmacological characteristics of flumazenil. Both Italian and international research articles have been selected, starting from 1980 to today.
Discussion and Conclusions: The use of flumazenil in slow infusion remains off-label but, for almost 20 years, it has been in use (in Italy and beyond) to detoxify, in about a week of hospitalization, patients with equivalent daily doses of diazepam greater than 50 mg / day for more than 6 months (with a description of cases up to 350 mg / day of diazepam-equivalent). This would allow the GABA-Argic receptor resensitization in a short time and with minimal or absent withdrawal symptoms, being able to suspend megadoses of BDZ in a very short time. In Italy this approach is still used to a limited extent and it cannot be said that it is absolutely the best method but the analysis of various endpoints in published studies, such as acute withdrawal discomfort, discharge without prescription of benzodiazepines and relapses in the first 6-12 months of discharge, certainly makes us reflect on the possibility of extending this approach to various territorial hospitals.
Benzodiazepines (BDZ) are among the most prescribed drugs in all European Union countries and, in Italy, it is estimated that chronic consumers, therefore off-label, are about 5% of the general population or about 3 million people [1]. In Italy they are classified as neuro-active, sedative-hypnotic drugs, of which there are about 30 active ingredients on the market with over 350 pharmaceutical packages (including generics) [2]. Because they are not reimbursable by the national health system as they are of category C, the consumption data are available only through sales. The OSMED 2021 report on the use of drugs in Italy has estimated that the average consumption of these drugs is over 50 DDD per 1000 inhabitants/day [3]. On average one in 10 adults uses it in life but the percentage rises to 1 in 4 in the over 65 age group (which is improperly done since, according to the BEERS and START / STOPP criteria, BDZs should not be used in elderly patients except when strictly necessary and at the minimum useful dosage) [4]. The prolonged use of these drugs tends to be addictive, with varying percentages depending on the studies, between 40 and 80%. The high prevalence of use derives from three factors: the multiple therapeutic indications (sedatives, hypnotics, anxiolytics, antiepileptics, muscle relaxants, pre-anesthetics), the high therapeutic index (BDZ intoxication alone hardly leads to death, contrary to barbiturates) and the demonstrated clinical efficacy in different contexts [5]. There are few true absolute contraindications (myasthenia gravis, severe heart or respiratory failure, pregnancy, advanced liver failure) but many relative ones, primarily age, co-intake of other sedative drugs, and a history of substance abuse [6]. The main side effects derive from an accentuation of the therapeutic ones, namely sedation, anterograde amnesia, somnolence, asthenia, ataxia, and reduction of cognitive performance [7]. Although many of these effects are tolerated by repeated administration, the use of these compounds in the elderly can lead to postural instability, an increased risk of accidental falls, and cognitive decline, although generally reversible [8]. BDZs are always drugs indicated for medium-short therapies, from a few days to a maximum of one or two months and not for long-term maintenance. In the context of long-term users (LTU), however, we can distinguish two types: - prolonged use at therapeutic doses: in Italy it would affect from 7.5 to 9% of the general population. All physicians, including primary care physicians, should apply slow escalations of medication until complete discontinuation [9].
- high dose users (HDU), in about 0.2% of the population but in 8-10% of BDZ users [10]. These are patients with developed tolerance and daily use of supratherapeutic doses, sometimes megadoses, in a state of apparent well- being but with significant withdrawal symptoms often interdose and dissolution or impossibility of progressive drug escalation [11].
In this article we want to describe how the use, off-label, of flumazenil under the skin in the slow infusion, can be useful as a therapy in the detoxification of these latter cases, HDU, analyzing the biochemical correlates and the data of studies carried out and so far available in Italy [12].
In this minireview, we conducted research in PUBMED and researchgate for papers on the biochemistry and use of flumazenil in BDZ abuse detox. We have selected research articles and revisions from 1990 to today, focusing in particular on data from Italian studies, with the primary endpoint to finding studies that spoke of the use of flumazenil not as an antidote in acute intoxication but in the detoxification of large abuse of BDZ. Secondary endpoints evaluated were the maintenance over time of abstention and the possible use in poly abusers, especially in the alcohol-BDZ co- association. We also used the technical data sheet of flumazenil itself and the PubChem database for chemicaltoxicological information.
Flumazenil (FLU, Figure 1, sold in Italy like solution for injection of 0.5 milligram/milliliter and 1 milligram/10 milliliter) is a competitive antagonist of benzodiazepines at the binding site of these drugs with the GABA-A receptor; this compound binds with very high affinity and selectivity central BDZ receptor and, with a competitive mechanism, prevents or interrupts the action of benzodiazepines with its binding site.9,10 The peculiar characteristic of this drug is therefore linked to the ability to prevent or antagonize all the effects induced by these drugs. Unlike benzodiazepines, FLU has no intrinsic activity, i.e. it is unable to activate the channel for GABA-dependent chlorine and, consequently, induce the same effects. Administered orally or intramuscularly, FLU has a low availability and therefore, to obtain a rapid pharmacological action, the preferred route of administration is the intravenous one. After an infusion, plasma concentrations are rapidly reduced in a logarithmic manner with a rapid half-life of 1-2 minutes (its antagonistic action lasts no more than two hours) and is then converted to metabolites eliminated by the kidney [11,12].
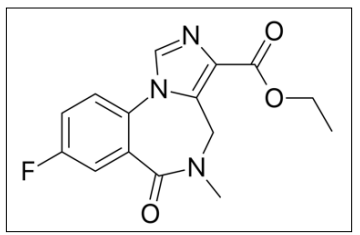
Figure 1A: Flumazenil Chemical Structure
Figure 1B: Flumazenil Pharmaceutical Form
Benzodiazepines, Z-drugs and barbiturates act on the GABA-A receptor complex, a pentameric structure composed of alpha, beta and gamma subunits that form a chlorine channel. These subunits exist in different subtypes and can be combined in various ways and the actions that result from binding with receptor agonists vary according to the composition of the subunits [13]. In particular, BDZ-sensitive receptors are composed of five subunits: two alpha, two beta and one gamma and the binding site is located at the interface of the alpha and gamma subunits. A histidine residue in alpha 1 and homologous residues in alpha 2,3 and 5 are essential for the action of BDZ [14]. Motor, cortical, in the pale globe, in the cerebellum and in the substantia nigra while in the limbic structures, including hippocampus, amygdala and anterior cingulate cortex, there is a prevalent concentration of alpha 5. Chronic administration of receptor agonists determines tolerance, more rapid for the hypnotic effects.. This phenomenon, for BDZ, is mainly attributable to the pharmacodynamic form and in particular to the desensitization and receptor down-regulation, to the reduction of the binding affinity with the compounds, to the reduced expression of mRNA for receptor proteins including AMPA and NMDA of glutamate [15].
The most frequent undesirable effects are of the gastrointestinal type, even though it is well tolerated. However, in patients with BDZ dependence and tolerance, acute flu administration has been associated with potentially severe withdrawal symptoms, including seizures [16]. Flumazenil is universally considered aBZD antagonist and administered as a bolus in the treatment of overdose. The interaction mode of binding with the receptor is shown in the Figure below.
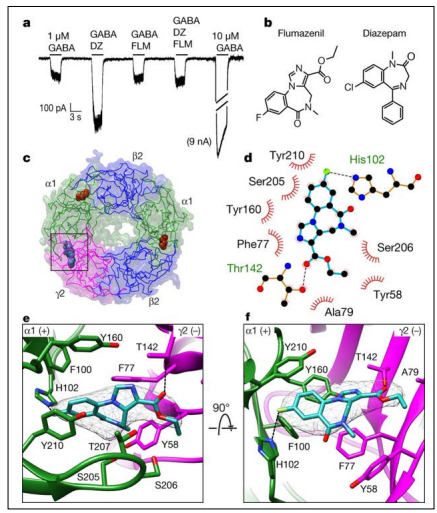
Figure 2: Flumazenil Interactions at the Benzodiazepine Binding Site
Experimental results have shown that FLU acts as a full agonist on
the alpha4 subunit of the benzodiazepine receptor, while acting as
an antagonist on the other isoforms, when administered by slow
infusion, with the following pharmacological actions:
• Alleviation of symptoms and signs of withdrawal
• Normalization and up-regulation of BZD receptors
• Restoration of the allosteric structure of the GABA-A receptorand inhibition of receptor uncoupling induced by BZD
• Inversion of tolerance
• Reduction of craving
• Limited relapse rates.
The variables that can affect the effectiveness of the FLU are: the
duration and dose of exposure to BZD: higher is the tolerance
and more effective the FLU is. In Italy, for some years now, the
Verona unit of Addiction Medicine applies the treatment with
slow infusion FLU in cases of chronic abuse of high doses of
BZD [17,18]. The treatment allows to suspend quickly (7-8 days)
very high doses of BZD (400 mg diazepam-equivalent, the median
dose daily abuse of some cases) well tolerated and with little
effect collateral [19]. The rapidity with which the slow infusion
FLU succeeds to re-sensitize the BZD receptors and the scarcity
of withdrawal effects led to the candidate for such treatment
becoming routine in detoxification from abuse of BZD. The
protocol developed by the addiction medicine unit of the Verona
polyclinic provides:
- A prophylactic antiepileptic therapy (with the use of valproic
acid in extended release formulation, “chrono”or levetiracetam
for intolerant patients)
- Hospitalization for one to two weeks, with suspension from
the first day of the abused BDZ and initiation of therapy with
subcutaneous FLU, diluted in physiological solution. Specifically,
patients were treated with a solution containing 7 mg of flumazenil
in an elastomeric pump (Figure 3), that was arranged with a
maximum capacity of 250 ml and constant release of 1.5 ml / h
for 7 days. The pump, releasing 1 mg of flumazenil every 24 h,
was then placed in a small bag that could be carried attached on
the shoulder [20].
- Possible use, for the first two-three days, of oral clonazepam
for the management especially of possible rebound insomnia. The
protocol provides clonazepam, orally administered every day in
the evening and gradually tapered from 6 mg on the 1st day to
0.5-2.0 mg on the last day of treatment.
- Optimization of the use of any adjuvants such as antidepressants
and non-narcotic drugs

Figure 3: a general type of elastomeric pump connected through a butterfly needle inserted into the anterior abdominal wall (from Slow subcutaneous infusion of flumazenil for the treatment of long-term, high-dose benzodiazepine users: A review of 214 cases. DOI: 10.1177/0269881116647505)
The antiepileptic prophylaxis has made it possible to reduce the risk of convulsive episodes to less than 2% of the cases treated and is then continued after discharge for 2-3 weeks while clonazepam is possibly used only for the first 2-3 days and then suspended. Main exclusion criteria are pregnancy, severe uncompensated mental disorders and untreated poly-abuse of other substances, as well as patients with less severe BZD dependence (daily intake of less than five times the DDD) [21]. The presence of medical comorbidities, including epilepsy and substance abuse under appropriate treatment are not exclusion criteria. In the case of comorbid alcohol and BZD dependence / misuse it is advisable to proceed with alcohol detoxification first (with BDZ), and consider longer FLU treatment (14-21 days). In a recent study by Lugoboni et al. Five male and nine female patients (mean age ± SD 42.5 ± 8.0 years) were hospitalized for BZD detoxification [22]. The use of BZD was discontinued on day 1 of admission. Therapy with any antidepressants was maintained and continued afterdischarge. All patients reported a history of BZD dependence according to DSM-5 criteria. Measurement of plasma levels of FLU and abused BDZ showed interesting results. Plasma FLU concentrations were 0.54 ± 0.089 ng / ml (mean ± SEM) at T1 after 4 days of continuous subcutaneous infusion, ranging from 0.14 to 1.4 ng / ml. Values recorded at T2 (end of therapy) were 0.09 ± 0.05 ng / ml, with FLU concentrations below limits of detection in 10 patients out of 14.Lormetazepam (LRM) levels were 502.5 ± 163.0 ng / ml at T0 baseline. A significant decrease (11.2 ± 5.7 ng / ml; p = 0.008) in LRM levels was recorded at T1 and 0.43 ± 0.43 ng / ml at T2. High LRM plasma levels recorded at T0 are in agreement with patients’ self-report of BZD use at admission, whereas low T1 and T2 levels with firm compliance to detoxification treatment. Lorazepam (LRZ) levels showed a similar pattern, with high initial plasma concentrations (83.1 ± 27.4 ng / ml), then a significant decrease to 20.4 ± 11.4 ng / ml (p = 0.01) at T1 and 9.4 ± 5.6 ng / ml at T2 after 7 days of FLU administration. Clonazepam (CLN) plasma levels were low at T0 (14.0 ± 8.6 ng / ml), 35.5 ± 5.0 ng / ml at T1, and 25.4 ± 3.9 ng / ml at T2. Note that three patients were treated with CLN before hospital admission. Another study evaluated the outcomes of 26 patients treated with low-dose flumazenil for benzodiazepine detoxification [23]. In particular 26 participants received low-dose subcutaneous flumazenil infusions (4 mg / 24 h for approximately eight days) as part of a randomized control crossover trial. Return to benzodiazepine use was assessed monthly for three months based on the benzodiazepine use in the previous week. Where data was not available, the treating psychiatrist examined patient files and clinical documents to determine benzodiazepine use [24]. Withdrawal and craving scores were also measured. The results were that abstinence rates from benzodiazepines at one-, two-, and three-month follow ups were 65.4%, 50.0%, and 46.2% respectively. When considering patient files and clinical documents for those lost to follow-up, abstinence rates were higher at 73.1%, 65.4% and 61.5% at the one-, two-, and three-month follow ups respectively. Withdrawal and craving scores were higher in those that had returned to any benzodiazepine use. Self-reported rates of abstinence from benzodiazepines at three months was between 46.2% and 61.5% [25]. Flumazenil may yield greater success than benzodiazepine tapering from high dose benzodiazepine use (≥30 mg diazepam equivalent). Further research should compare abstinence rates after treatment with flumazenil compared to benzodiazepine tapering in high dose benzodiazepine users. In addition to benzodiazepines, the subcutaneous treatment with FLU in slow infusion has also been proposed for the detoxification from abuse of “Z-drugs”, in particular from zolpidem (which is the most sold in Italy). Already in 2005 Lugoboni et al. have published a case report on two patients detoxified effectively by high doses of zolpidem with the same procedure performed in the context of the BDZ with an antiepileptic prophylaxis and an average hospitalization of one week, with a favorable course and without relapse. In fact, it should be remembered here that zolpidem (Figure 4) is chemically an imidazopyridine, with a higher binding affinity for some GABAergic receptor subclasses but functionally it behaves as an agonist like the classic BDZ [26].

Figure 4: Zolpidem Chemical Structure
In 2003 Gerra et al. published a randomized, placebo-controlled study of Intravenous flumazenil versus oxazepam tapering in the treatment of benzodiazepine withdrawal. Flumazenil (FLU) was previously found unable to precipitate withdrawal in tolerant subjects submitted to long-lasting BZD treatment [27]. In the experiment, FLU (treatment A) was compared with oxazepam tapering (treatment B) and placebo (treatment C) in the control of BZD withdrawal symptoms in three groups of BZD dependent patients. Group A patients (20) received FLU 1 mg twice a day for 8 days, and oxazepam 30 mg in two divided doses (15 mg + 15 mg) during the first night, oxazepam 15 mg during the second night and oxazepam 7.5 mg during the third night. FLU was injected i.v. in saline for 4 hours in the morning and 4 hours in the afternoon, in association with placebo tablets. Group B patients (20) were treated by tapering of oxazepam dosage (from 120 mg) and with saline solution (as placebo) instead of FLU for 8 days. Group C patients (10) received saline instead of FLU and placebo tablets instead of oxazepam for 8 days [28]. FLU immediately reversed BZD effects on balance tasks and significantly reduced withdrawal symptoms in comparison with oxazepam and placebo on both self-reported and observer-rated withdrawal scales [29,30,31]. The partial agonist also reduced craving scores during the detoxification procedure. In addition, during oxazepam tapering, group B patients experienced paradoxical symptoms that were not apparent in FLU patients. Patients treated with FLU showed significantly lower relapse rates on days 15, 23 and 30 after the detoxification week. Our data provide further evidence of FLUs ability to counteract BZD effects, control BZD withdrawal and normalize BZD receptor function [32,33]. The effectiveness of FLU may reflect its capacity to upregulate BZD receptors and to reverse the uncoupling between the recognition sites of BZD and GABA, on the GABA (A) macromolecular complex that has been reported in tolerant subjects.
The use of Flumazenil in the detoxification of high dose abusers of BDZ is not a recent procedure as it has been applied in some clinics including in Italy for over 15 years. To date, we cannot say that this is the absolute best methodology for obtaining and, above all, maintaining over time the total abstention from BDZ in subjects who use at least 50 mg / day of diazepam-equivalent [34,35]. Certainly, the results available to date say that, compared to other methods such as progressive dose scaling or replacement with long acting agonists like clonazepam (very effective methods in therapeutic dose users), which have often proved unsuccessful in this type of patients, are often unsuccessful in high dose users [36]. Subcutaneous FLU, after antiepileptic prophylaxis, allows the clinical to quickly suspend (in about 7-10 days) high doses of BDZ or Z-drugs with a seizure risk of less than 2% and without excessive withdrawal symptoms, controllable with use in the first 2 -3 days of low doses of symptomatic drugs [37,38]. Unfortunately, in Italy, this method has not been the subject of extensive studies over the years and, to date, it is applied only to the polyclinic of Verona [39,40].
Competing Interests: The authors declare that they have not had any conflicts of interest or external sources of funding
Author Contributions: All the authors contributed to the writing of the article; E. Marovino had the idea of writing the article, supervised the work and wrote the introduction and conclusions while A. and C. Morgillo, together with A. Cambareri, researched the bibliographic sources and elaborated the part of the discussion.
