Author(s): Sena Aksoy*, Mina Uzülmez and Aysun Soysal
Voltage-gated potassium channel (VGKC) encephalitis is a type of autoimmune encephalitis, that presents with memory impairment, headache, psychiatric symptoms and seizures. Although contactin-associated protein 2 (CASPR2) and leucine-rich glioma inactivated 1 (LGI1) are clearly identifed as components of the VGKC complex, additional subtypes are known to exist. In this report, we present a case of 64-year-old patient with VGKC antibodies, negative for CASPR2 and LGI1.
A 64-year-old male patient admitted to emergency department of our hospital, with the complaints of headache and memory loss. He had been suffering from headache for three months, more pronounced during two weeks before admission. His family described a period of about two weeks, in which he experienced fluctuated memory loss, by asking the same questions, sometimes in inappropriate way. There was no history of any chronic disease or medication use. He had no known personal or family history of any neurological or psychiatric disorders.
His first physical and neurological examinations were normal. Cranial computed tomography (CT) scan and diffusionweighted magnetic resonance imaging (MRI) did not reveal any abnormalities. Because of analgesic-resistant headache, he was examined with brain MRI with contrast, that showed T2-FLAIR hyperintensities in medial and hippocampal structures of both temporal lobes, extending to left insular cortex, and heterogenous contrast enhancement in left medial temporal lobe (Figure 1). The patient was hospitalized for further investigation.
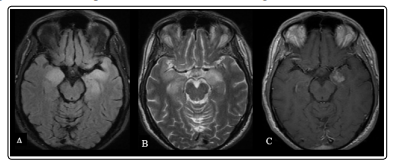
Figure 1: Brain MRI at the time of admission, showing bilateral T2-FLAIR hyperintensities in medial temporal lobes (A,B) and contrast enhancement in left medial temporal lobe (C).
The initial investigations such as complete blood count, thyroid, liver and renal function tests, vitamin B12 level, C-reactive protein and erytrocyte sedimentation rate were all normal. Due to differential diagnosis consisting of viral encephalitis, limbic encephalitis and brain tumors, lumbar puncture was performed. In cerebrospinal fluid (CSF) examination, 40 lymphocytes were found with protein level of 70,94 mg/dl and glucose level of 56,7 mg/dl while the level of blood glucose was 120 mg/dl. Acyclovir treatment (30 mg/kg/day) was started as viral encephalitis could not be ruled out, although CSF herpes simplex virus PCR test was negative. Other encephalitic etiologies such as toxic, metabolic and infectious were considered and ruled out. CSF samples. for pathological examination of atypical cells, the examination of antibodies against N-methy-D-aspartate (NMDA-R), α-amino3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA-R1, AMPA-R2), voltage-gated potassium channel complex for contactin-associated protein 2 (CASPR2) and leucine-rich glioma inactivated 1 (LGI1), gamma-aminobutyric acid (GABA-R) and blood samples for the examination of antibodies against Hu, Yo, Ri, amphiphysin, Tr, PCA-2, Ma, CV2-1, ANNA-3 were done and found to be negative. Oligoclonal band was Type-3 pattern positive, with the IgG index of 0.8. To investigate metastatis, thoracal and abdominal CT scan were performed and showed no evidence of an occult tumor, moreover tumor markers such as CEA, CA 15-3, CA 19-9 were in normal range. Positron Emission Tomography (PET)/CT was performed to find out whether the patient had cancer and showed mildly increased fluorodeoxyglucose (FDG) uptake in right medial temporal region of brain, whereas no pathological uptake was found in the body. Electroencephalography (EEG) was also found normal.
After giving acyclovir for 14 days, lumbar puncture was repeated to check the response to treatment. CSF analysis showed 20 lymphocytes, with protein level of 87,1 mg/dl and glucose level of 55,3 mg/dl while the level of blood glucose was 108 mg/dl. Because improvement in clinical findings was not enough, pulse steroid (1000 mg/day for 7 days) was given with the following oral metylprednisolone (1 mg/kg/day), considering the diagnosis of limbic encephalitis, although any related antibody could not be found. After intravenous steroid treatment, both clinical and radiological responses were obtained and the patient was discharged.
Three months later, the patient admitted to hospital with headache and disorganized behaviors after stopping oral steroid one month before admission. In his neurological examination, orientation in time was disturbed. Brain MRI revealed T2-FLAIR hyperintensities in both temporal lobes, extending to left insular cortex, and increased contrast enhancement in both temporal lobes extending to left insular cortex, comparing to his previous MRI (Figure 2). Lumbar puncture was performed and showed 30 lymphocytes, with protein level of 102,89 mg/dl and glucose level of 59,9 mg/dl (simultaneous blood glucose was 109 mg/ dl). Oligoclonal bands were found as Type-3 pattern positive and IgG index was 0.6. Because central nervous system lymphoma was in the list of differential diagnosis, flow cytometry test was examined in both serum and CSF samples and no abnormal/clonal lymphocyte population was found. EEG was repeated and resulted as normal. The patient was transferred to neurosurgery department with the plan of biopsy from right temporal region. Meanwhile, the patient developed tremor in both hands and his confusion was progressed, with limited cooperation and disturbance in time and place orientation.
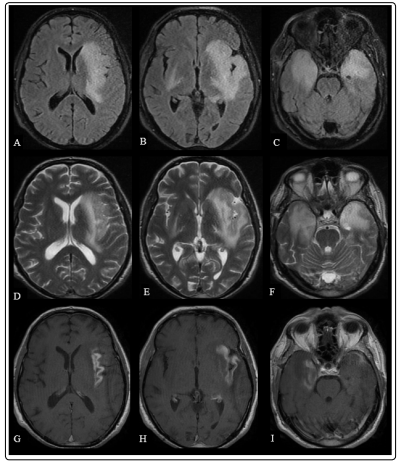
Figure 2: Brain MRI showing bilateral T2-FLAIR hyperintensities in medial temporal lobes, left frontal lobe and insular cortex (A,B,C,D,E,F) and contrast enhancement in right medial temporal lobe and left insular cortex (G,H,I).
One month after surgery, because his symptoms were persisted, he was given pulse steroid for 10 days, followed by oral methylprednisolone. The pathological examination indicated inflammatory process leading to myelin destruction, no evidence supporting neoplastic or granulomatous lesion. Because neoplastic and infectious etiologies were ruled out, lumbar puncture was repeated to examine antibodies causing autoimmune encephalitis and resulted as negative. Despite this fact, IVIg (2 gr/kg per 5 days) was given for limbic encephalitis and serum sample of the patient was sent to another laboratory for the investigation of antibody against VGKC complex and found as positive (115 pmol/L). Thereby, the diagnosis of autoimmune encephalitis was confirmed and the treatment protocol as IVIg (1 gr/kg/month) and rituximab (500mg/m2, followed by a second dose 2 weeks later) was started. No new signs/symptoms was seen after treatment so far, moreover, control imaging with MRI showed resolution in lesion in insular cortex (Figure 3). To rule out malignancy, an annual PET examination was planned.
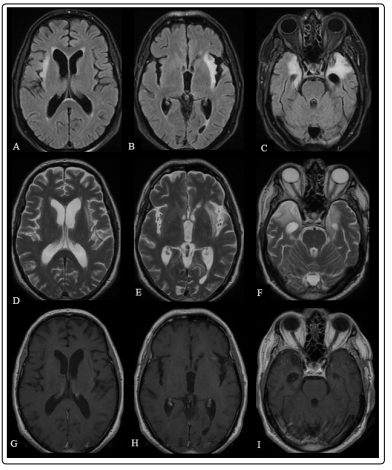
Figure 3: Brain MRI after treatment with IVIg showing bilateral T2-FLAIR hyperintensities in medial temporal lobes and insular cortex (A,B,C,D,E,F) and decreased contrast enhancement in insular cortex comparing to previous MRI (G,H,I)

Figure 4: Timeline indicating treatment protocol given to the patient
Autoimmune encephalitis is relatively rare and new group of neuroinflammatory disorders. The clinical findings include a variety of symptoms such as altered mental status, headache and seizures, depending on the antibody involved [1, 2].
Antibodies against the VGKC complex is the second most common cause of autoimmune encephalitis after anti-NMDAR encephalitis and it has a wide range of clinical manifestations such as limbic encephalitis, Morvan syndrome and neuromyotonia [3,4]. Their pathogenic roles in central nervous system was first described in 2001 [5]. Voltage-gated potassium channel encephalitis is mostly characterized by memory impairment, psychiatric symptoms and seizures [1-3].
According to recent studies from the literature, VGKC encephalitis is generally presented by T2-FLAIR hyperintense lesions on one or both medial temporal lobes on magnetic resonance imaging of the brain, besides postcontrast enhancement in some patients, which is consistent with our case [1,7]. Furthermore, in many cases described in the literature, CSF pleocytosis was observed. For this reason, differential diagnosis should include infectious, toxic, metabolic encephalitic etiologies and intracranial tumors [6]. Another thing to remember that all diagnostic work-up should be carefully done to exclude other reasons which may cause similar symptoms, because the exclusion of alternative causes is still accepted as a diagnostic criteria [7].
VGKC complex is composed of contactin-associated protein 2 (CASPR2), leucine-rich glioma inactivated 1 (LGI1), dipeptidylpeptidase-like-protein 6 (DPPX) and VGKC with unknown antigen [2, 8]. It is clearly known that the majority of high-titer VGKC antibodies are directed against LGI1 and CASPR2 [3,9]. However, the clinical importance of low-titer antibodies still remains unclear. Anti-VGKC titers with negative anti-LGI1 and anti-CASPR2 are typically low ( <0.3 pM) and there is still a debate on whether they are responsible for immune-mediated pathogenesis [7]. In our case, considering clinical features compatible with VGKC encephalitis, MRI and laboratory findings, and response to immunotherapy, low-titer antibodies can be responsible for the clinical picture.
To date, the treatment of autoimmune encephalitis is immunotherapy, irrespective of the type of antibody. It is claimed to start immunotherapy with intravenous steroid followed by oral steroid maintenance therapy as the first-line therapy. If it is ineffective or the symptoms are severe at the onset, intravenous immunoglobulin or plasma exchange should be considered [8,10]. For the second-line therapy, rituximab and cyclophosphamide are the mostly-used therapeutic agents. Although VGKC encephalitis is associated with malignancy in minority of the cases, considering the diagnosis of cancer after the detection of VGKC antibodies, the evaluation of patients for possible malignancy is suggested [4,8].
VGKC encephalitis is one of the most common autoimmune encephalitis. Given the broad spectrum of VGKC autoimmunity and the wide variety of clinical manifestations, there is a need to understand additional subtype specificities and better treatment modalities.
